Electrophysiologically Guided Multitarget Stereotactic Intractable Epilepsy Surgery in Patients with Complex Epileptic Systems
- 1. Department of Neurology, The University of Chicago, Chicago, USA
- 2. Department of Functional Neurosurgery, Epilepsy Surgery Center, The Saradzhishvili Institute of Clinical and Experimental Neurology, Tbilisi, Georgia, USA
Abstract
Objective: The purpose of this study is to achieve beneficial treatment outcomes for severe intractable epilepsy patients using neurophysiologically guided stereotactic multitarget surgery.
Material and methods: Ninety-three patients (64 men, mean age 25 y (SD – 11 y, range 6-57 y), mean duration of illness 18 y (range 3-36 y) underwent multitarget stereotactic cryosurgery guided by pre- and intraoperative depth electrode (stereoelectroencephalography – SEEG) evaluation. Multiple unilateral and bilateral amygdalatomies, partial anterior and total hippocampotomies, cingulotomies, fornicotomies, CM and DM thalamotomies, postero-medial hypothalamic, Forel-H-tomies, and fasciculus uncinatus lesions in individual combinations were performed according to SEEG findings.
Results: The SEEG studies revealed the existence of complexly organized multistructural epileptic systems in cases of long-standing severe intractable epilepsy. Engel’s (1993) Class I outcome was achieved in 51%, worthwhile improvement (Classes II-III) was observed in 28% and no worthwhile improvement (Class IV) was observed in 21% of all patients. Remarkable normalization of the psycho-emotional state was achieved for patients with pre-surgical behavioral problems. No seizure, or cognitive, or memory states worsening was observed in this cohort of patients. The follow-up for seizures and behavioral abnormalities was up to10 years.
Conclusion: Multitarget electrophysiologically guided stereotactic surgery can have a beneficial effect on seizure frequency and severity, and normalize psycho-emotional state and behavior in long-standing intractable epilepsy patients. We did not postsurgical decline in cognitive domain of our patients, and the benefits of seizure control using this technique, im our opinion,outweigh possible risk of cognitive decline.
Citation
Chkhenkeli SA, Lortkipanidze GS, Rakviashvili TN, Magalashvili GE, Bregvadze ES (2013) Electrophysiologically Guided Multitarget Stereotactic Intractable Epilepsy Surgery in Patients with Complex Epileptic Systems. J Neurol Transl Neurosci 2(1): 1030.
Keywords
• Complex epileptic systems
• Intractable epilepsy
• Neurophysiologic guidance
• Psycho-emotional disturbances
• Stereotactic multitarget epilepsy surgery
INTRODUCTION
According to widely accepted criteria, the potential candidates for resective intractable epilepsy surgery should have a detectable epileptic focus localized outside of the eloquent cortical areas and, in cases of temporal lobe epilepsy, within one temporal lobe. Adherence to these criteria leaves no hope for a large group of disabled patient with severe intractable epilepsy and epilepsy-induced psycho-emotional disturbances, and limits the cohort of potential candidates for successful epilepsy surgery. A multicenter study [1] demonstrated that 30% of patients who underwent presurgical evaluations for resective epilepsy surgery ultimately did not have surgery because of multifocality of seizures, localization of epileptic focus (foci) within eloquent cortical areas, or the risk of severe postsurgical memory impairment. For these patients, leaving seizures uncontrolled may result in further decline of speech, memory, learning, emotional stability, or cognitive and psychosocial dysfunction, leading to dependent behavior and a restricted lifestyle.
However, localization or approachability of an epileptic focus is not the only limitation. Contemporary epilepsy surgery is directed mainly against a solitary epileptic focus whereas intractable epilepsy may be considered as a dynamic multifactoral process with complexly and multistructurally organized epileptic networks [2-15]. Conventional resection of most active elements of these epileptic networks is hard to perform, but stereotactic method offers a possibility to conduct simultaneous surgery on the key elements of epileptic network. The outcomes of the previous stereotactic surgeries with small lesions targeted also to the sole epileptic focus or neural pathway were not found to be as favorable as those obtained with standard temporal resections [16]. To summarize the existing experience with stereotactic lesional treatment of epilepsy, it is necessary to understand that there are particular reasons that lead to the failure of stereotactic method for epilepsy treatment. In many clinics, these surgeries have been performed using “standardized” operations, without detailed detection of the “architecture” of the pathologic intracerebral network (epileptic system), without detailed neurophysiological analysis of the interrelations between key elements of these epileptic systems, and without modification of surgeries according the needs of each individual patient. Furthermore, it could be that not all key elements of the epileptic system were lesion allowing the remaining parts to transform and continue their activity if left intact. Our experience suggests neurophysio;ogically guided precise stereotactic surgery, which impacts key multitarget elements of the epileptic systems, may frequently lead tp reorganization and normalization of the brain activity resulting in successful clinical outcomes.
PATIENTS AND METHODS
Patients
This study included a highly selected cohort of 93 long-standing intractable epilepsy patients (64 men, mean age 25 y (SD- 11 y, range 6-57 y), mean duration of illness 18 y (SD- 9.63, range 3-36 y), and the frequency of seizures occurrence ranged from 6 to 70 per month. Most of these patients were clinically defined as intractable temporal lobe epilepsy patients with a likelihood of complexly organized epileptic systems, including limbic-thalamic structures. Seizure manifestations included complex partial seizures with and without secondary tonic-clonic generalization, “primary” generalized seizures with elements of psychomotor seizures. Most of the patients were additionally incapacitated by psycho-emotional and behavioral disturbances (Tables 1 and 2). Multiple presurgical scalp EEGs, long-term video-EEG monitoring and telemetric EEG recordings revealed bitemporal and multifocal independent, as well as bilateral synchronized interictal and ictal epileptiform abnormalities (Table 3).
The patients we have studied have been divided into two groups, A and B, different from each other by the degree of neurophysiologic analysis of the clinico-EEG/SEEG data and by the number and volume of stereotactic lesions. Group A included 31 patients (39 surgeries) whose EEG/SEEG data were assessed only from the point of view of localization of the putative epileptic focus. In this group, the goal of the patient’s evaluation was to detect a restricted epileptic focus, supposedly responsible for the full clinical set of symptoms, and stereotactic lesions were limited in number and the size of the lesion according to existing surgical practices.
Group B consisted of 76 patients (62 patients + 14 patients from Group A with unsatisfactory surgical outcome who underwent reoperation) included in Group B were operated on using multitarget electrophysiologically guided lesioning of the key elements of the individually organized epileptic systems. The extent of surgery was planned according to the results of the pre-and intrasurgical investigation in each particular patient. The age, clinical, electrophysiological, CT, MRI, and neuro-psychological status of patients in Group A and Group B were similar, and their treatment outcomes were comparable.
Pre-surgical evaluation
As a rule, AEDs were temporary reduced, and at least two spontaneous seizures documented by long-term video/EEG, video/telemetric EEG/SEEG monitoring were required during the pre-surgical evaluation. In the assessment of the patients psychoemotional state, attention was focused on the interictal, immediately preictal and postictal manifestations. The neuro-psychological battery included the adapted Wechsler (WAIS & WISC) Scales, TAT, MMPI and Rorschach tests. Patients’ evaluations revealed different degrees of the temporo-limbic system involvement with putative lateralization in some cases most patients had an IQ ranging from low-average to average, exhibited both verbal and nonverbal memory difficulties, indicating bitemporal dysfunction, and displayed interictal psychotic profiles on the MMPI. To assess memory, we selected a number of the most frequently occurring common nouns, paying particular attention to their length (max. 2-4 syllables). In the memory examination, during the one tesr the patient was presented with series of ten words and a short (5-6 word) sentence presented verbally twice. The second test included ten word lists and series of material that cannot verbalized readily, such as places, unfamiliar faces, or abstract designs and drawings presented visually for one minute. Memory assessment was based on the ability of patients to reproduce presented material after five minutes.
Decision making
The results of neurologic, EEG, CT, and MRI evaluations in this cohort of patients, especially in the Group B patients, were inconclusive about the site of seizure origin. The results of the assessment of clinico-neurophysiologic data, including neuro-psychological assessments, served as the basis for an elaboration of the preoperative hypotheses about the organization of the putative individually organized epileptic system and indications for invasive SEEG-evaluations for the detection of the key elements of these systems. Concurring with the statement that a proposed operation for an epileptic patient cannot be safely based on a general hypothesis, and should only rest on knowledge of the functional organization of the epileptic system, we did not make standardized preoperative decisions about the extent of surgery. The final decision about the lesioning of specific brain structures involved in the individual epileptic system was made during surgery, and was based on the cumulative assessment of the pre- and intrasurgically obtained information.
Table 1: Clinical manifestations of seizures.
| Types of epileptic seizures* | Number of patients |
| Complex partial seizures (CPS) with frequent secondary fast or delayed generalization | 42 |
| Clinically “primarily” generalized seizures with “postictal” automatisms | 34 |
| CPS or “primarily” generalized seizures with postictal lateralizing neurological deficit | 31 |
| CPS with postictal twilight states | 23 |
| Clinically “primarily” generalized seizures with elements of partial motor seizures | 19 |
| Drop-attacks-like seizures with subsequent tonic stiffening | 17 |
Types of seizures are described not just according to Classification but with important clinical and behavioral phenomenology. Most of patients exhibited more than one type of seizures.
Table 2: The main clinical manifestations of the psycho-emotional and behavioral disturbances.
| Types of the psycho-emotional and behavioral manifestations* | Number of patients |
| Interictal chronic depression | 43 |
| Interictal hypersexuality** | 13 |
| Interictal acute psychotic states concomitant with “forced normalization “ of EEG | 11 |
| Interictal emotional excitement, anxiety | 23 |
| Preictal changes of mood, irritability, fear, explosiveness, and anxiety | 52 |
| Postictal fear and/or anxiety | 14 |
| Postictal psychotic states, anger attacks, excessive hypersexual behavior | 12 |
* Table 3 mirrors the main types of the psycho-emotional and behavioral manifestations. Many of patients demonstrated more than one pattern of an abnormal psycho-emotional state and frequent transformation of one psycho-emotional state into another.
** We do not describe hyposexuality, which is common for temporal lobe epilepsy and less disturbing to everyday life.
Table 3: EEG characteristics of the scalp video-EEGs or telemetrically captured seizures.
| EEG patterns of seizures* | Number of patients |
| Temporal unilateral with or without generalization | 13 |
| Temporal unilateral → contralateral temporal mostly with generalization | 22 |
| Temporal unilateral → contralateral fronto-parietal with or without generalization | 13 |
| Bitemporal independent with or without generalization | 25 |
| Bitemporal bilaterally synchronous mostly with instantaneous generalization | 8 |
| Temporal unilateral → ipsilateral frontal → contralateral frontal with generalization | 11 |
| Temporal unilateral → bilateral frontal with generalization | 7 |
| Multifocal (mostly anterior frontal or posterior temporal) with generalization | 14 |
| “Forced normalization “ of EEG with “primary” generalized seizures** | 10 |
| Temporal lobe electrodecremental event → temporal ipsilateral with generalization | 31 |
| Diffuse electrodecremental event with “primary” generalization | 27 |
* This Table presents the main electrographic abnormalities, which were dominant in the recorded interictal and ictal EEGs. Arrows indicate a direction of seizure spread detectable in the EEG and chronic SEEG.
** Forced normalization of EEG could be observed in patients with focal temporal epileptic focus on EEG, bitemporal abnormalities, as well as, in patients with multifocal and diffuse epileptiform activity.
SURGERY
Surgery, methods
Stereotactic operations were performed using Talairach’s stereotactic frame. Electrode insertion was usually performed under local and neurolept anesthesia with N2 O + O2 ventilation. Subsequent intrasurgical diagnostic studies and lesions were performed in extubated awake patients receiving local anesthesia. Temporal lobe mesiobasal structures were located using an axis of reference constructed on the temporal horn fiducially points [17]. Amygdala and hippocampal structures and exact locations of the intracerebral electrodes were defined by intrasurgical orthogonal televentriculography using water-soluble contrast agents. Thalamic, subthalamic, and hypothalamic structures were reached by coordinates related to AC-PC line, saggittal midline, and a proportional grid according to thalamic size. The SEEG electrodes and lesional tools for evaluation/lesion of the thalamo/subthalamic structures were usually inserted using tangential approach. The cingular, fornical, anterior commissure, and temporal lobe instruments were usually inserted through a lateral approach [17].
Surgery, targeting
Hippocampus: In several cases, we used a posterior longitudinal approach to the hippocampus, but our study demonstrated that this approach does not always allow to reach a whole hippocampal volume using just two fiducially points: entry point and uncus [18]. That is the reason why we prefer the lateral approach to different parts of hippocampus. For a “total” hippocampotomy on the side of putative dominant epileptic focus, we usually performed three lesions of different volume, intending to maximally include the intraventricular part of structure as corresponding to the CA1-CA3 fields of the cornu Ammonis [19]. The epileptic focus activity recorded by each SEEG electrode’s five contacts determined the volume of the lesion. Anterior hippocampotomy was limited to the head of hippocampus, including its intraventricular part, the digitationes hippocampi, and an extraventricular or uncal part primarily targeted on the inferior and medial part of CA1 (Sommer) sector as most vulnerable part of hippocampus. The CA1 sector of hippocampus is a source of hippocampo-cortical output to the prefrontal and orbito-frontal cortex [20,21] and appears to be an important target for surgery.
Fornicotomy: Pursuing the goal to perform total hippocampotomy (stereotactic “hippocampectomy”), we usually performed a fornicotomy ipsilateral to the subtotal hippocampotomy in the compact part of the fornical columns at the level of anterior commissure to prevent the possible spread of epileptic activity from the remaining posterior part of hippocampus to the mamillary body, thalamus, and cortex.
Amygdala: A total amygdalatomy was usually performed in isolation, or on the side of dominant epileptic focus and total hippocampotomy. Contralateral amygdalatomy, when it was performed, was usually centered on its basal, lateral, and central nuclei which have limbic function and output to the dorsomedial thalamic nucleus, and then to the prefrontal cortex, as well as to the lateral hypothalamus and tegmental area. The right amygdalatomy usually was performed slightly larger than left, because of the interhemispheric asymmetry of human amygdalas [22,23].
Cingulum. Anterior cingular cortex (field 24 of Brodman) and cingulum bundle: Cingulotomies were performed to remove both anterior cingulate cortex and the cingular bundle in cases with apparent involvement of anterior cingular area in seizure spread. Intraoperative cerebral angiography was used for the precise targeting of the limbic part of the gyrus cunguli located between callosal and calloso-marginal sulci and for preventing hemorrhagic complications. Calloso-marginal sulcus is often doubled, and more frequently, it is doubled in the right hemisphere. In such cases, the specifically limbic cortex is limited to the internal segment of gyrus. The secondary branches of the A2 segment of the anterior cerebral artery very well outline these anatomical peculiarities. Besides that, the diameter of the left A2 is bigger, and the difference in diameters can be about 0.2-5.0 mm. The intraoperative angiography allows the precise targeting of the limbic cortex, as well as avoiding hemorrhagic complications [24]. Special attention was given to the lesion extent in the coronal plane, because it has been stated that sometimes the lesion might not involve the cingulum bundle [25].
Forel-H- fields. Campotomy: Campotomy was performed in the cases of fast frontal and prefrontal seizure spread and motor generalization to intercept the descending impulses and elevate the threshold of motor structures in order to reduce or avoid the clinical tonic-clonic seizure component [26]. The Forel-H- fields was targeted in cases with apparent involvement of this area in seizure spread and was centered on the prerubral area, aiming at the H3 field uniting H1, H2 fields and zona incerta, which receives prefrontal motor afferents. Cryogenic lesions in this area never exceed 4mm in diameter.
Postero-medial hypothalamotomy: Postero-medial hypothalamotomy was performed in patients with seizure-related aggressive behavior and hypersexual abnormalities, and SEEG verification of hypothalamic involvement into the seizure discharge propagation. The 4-5 mm diameter target was chosen according to Sano [27] and was located 1 mm anterior and 3-4 mm inferior to the CA-CP line midpoint, 1-3 mm lateral to the wall of third ventricle.
The fornicotomies, cingulotomies, Forel-H-tomies, and postero-medial hypothalamotomies were performed not as single-target epilepsy surgeries as it was introduced by their authors, but as lesions of important epileptic system parts performed simultaneously with lesion of dominant epileptic focus (foci).
Surgery, SEEG evaluation, functional probes
Intracerebral electrodes for chronic and intrasurgical SEEG evaluations and functional probes with direct stimulation, local polarization and cooling of deep brain structures were described earlier [28]. EEG/SEEG recordings (DC-80 Hz bandpass) were obtained with a 20-channel Alvar recording system (Alvar-Electronic, France). Local diagnostic bipolar stimulations (usually 0.5-5.0 mA, 0.1-0.2 ms, 0.5-1.0 s) were performed using Nihon-Kohden (Tokyo, Japan) stimulators and constant-current square pulses of alternate polarity with parameters chosen to avoid tissue damage [29]. The pharmacological provocation and augmentation of focal epileptic activity was achieved with i. v. administration of 50 -100 mg Brevital (Metohexital) and 25 mg/20 s Bemegride (Megimide) until the emanation of epileptic focus activity [30].
The temporary reversible “shut-off” of deep brain structures was achieved with local reversible cooling and/or local low-intensity (0.5-1.0 mA) anodic polarizations. This allowed us to evaluate the interrelations of the epileptic system elements and avoid the postsurgical activation of the previously less active brain structures after lesion of the dominant focus [3,28,31].
The intraoperative study protocol consequently included: 1) recording of interictal electrical activity, spontaneous focal subclinical and spreading epileptic activity; 2) diagnostic electrostimulation of the elements of putative epileptic system; 3) reversible “shut-off” of active elements of these systems; 4) pharmacological augmentation and provocation of epileptic activity and discharges. Each next step in this protocol was performed 5-10 min after returning the SEEG/EEG activity to the baseline. To prevent clinical seizures, 10 mg Valium was usually administered to the patient after the final pharmacological stage of study.
During independent assessment (SCh, GL, and ShB) of the SEEG/EEG data, the most important patterns were: 1) absence of spontaneous epileptic activity; 2) focal intermittent epileptic activity or discharges in one of the recorded structures; 3) spread of this epileptic activity to brain structures of same anatomical/ functional level (i. e. amygdalar activity to the hippocampus and vice versa) ; 4) spread of epileptic activity beyond the lobar limits of one hemisphere (i. e. spread of amygdala-hippocampal activity to the homolateral frontal lobe) ; 5) involvement of symmetrical contralateral structures; 6) spread of deep brain activity to the contralateral scalp EEG; 7) the sequence of discharge spread and generalization; 8) temporary focal suppression of activity in one of brain structures during a focal subclinical seizure in another, or augmentation of epileptic activity during the temporary “shut-off” of an epileptic focus.
Surgery, lesioning
The electrophysiological criteria for lesioning were: a) prevalence of interictal activity from one side, obvious and reiterative following changes in interictal activity in one temporal lobe to changes in the temporal lobe with a prevalence of spike activity; b) stable onset of subclinical and clinical seizures from the same temporal lobe; c) stereotyped initial clinical manifestation of seizures; d) apparent unilateral CT, MRI, and positive ventriculography changes. Additionally, the mutually suppressive interactions of hippocampal epileptic foci heralding possible activation of another hippocampal epileptic focus after the ablation of one of them [28] served as an indication for bilateral hippocampal surgery. Cryolesions (freezing) of the epileptic foci tissue were performed using a portable cryosurgical device producing precisely calibrated and volume-controlled lesions [32].
POST-OPERATIVE EVALUATION AND FOLLOW-UP
Postoperatively, the EEG and neuropsychological status of all patients were evaluated twice during their two-week hospital stay; 87 patients were evaluated in 3 and 6 m, 78 - after one year, 53- after two years, 31- after five years, and 17 patients after 10 years of surgery. Additional multiple EEG evaluations were performed in between these established times. Postsurgical changes in intellectual, memory, and language were additionally assessed based on self-reports, as well on the reports of family members.
All 93 patients were evaluated and operated on at the Center of Functional Neurosurgery and Epilepsy Surgery of The Institute of Clinical and Experimental Neurology, (Tbilisi, Georgia).
The experimental protocol was approved by the Institutional Medical Council (an analogy of the Institutional Review Board) with written informed consent being obtained from all patients or their guardians.
RESULTS
The outcomes of surgery in Group A patients were in general not as good as expected. The exception was a considerably better outcome in five patients who received an additional stereotactic amygdalatomy with partial anterior hippocampotomy contralateral to the previous unsuccessful anterior temporal lobectomy because of activation of the contralateral temporal lobe epileptic focus after their first surgery (Table 4).
Meticulous analysis of the already performed surgeries results and growing clinical and SEEG data revealed the complicated interrelations between the ipsi- and contralateral brain structures, and variable paths of seizure spread and generalization in our cohort of patients.
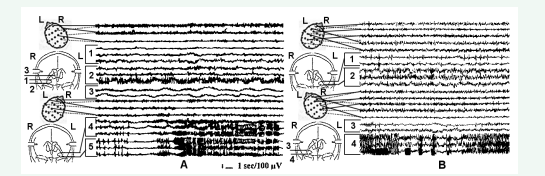
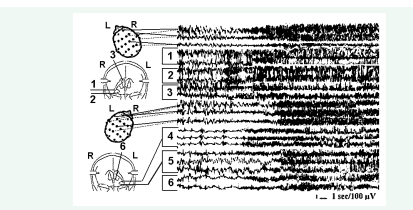
Accordingly, our goals for patients’ evaluation and surgery were expanded. The pre- and intraoperative evaluation goal appeared as detection of the most active elements of the epileptic system, evaluation of the variants of their interrelations and pathways, and the consequence of epileptic discharge spread in each individual patient. The deep electrode studies revealed the different variants of architecture of the epileptic systems and spread of epileptic discharges in intractable epilepsy, which influenced the surgical strategy and outcome. First, it was found, at least in our cohort of patients, the almost constant bilateral involvement of amygdala-hippocampal complexes in the epileptic process. A strictly unilateral mesiobasal epileptic focus was found in 17% (16/93) of cases. For the remaining 77 patients, seemingly bilateral interictal and ictal epileptic activity was assessed as predominantly unilateral in 19% (18/93) of cases. In all other cases (59/93, 64%), the interictal as well as spontaneous ictal epileptic activity revealed the bilateral, mostly independent seizure onset and involvement of temporal lobe mesiobasal structures in the epileptic process. The degree of this involvement differed, including continuous or intermitted interictal epileptic activity in both hippocampi, spontaneous subclinical seizures in the one amygdala-hippocampal complex and persistent interictal epileptic activity in the contralateral structure with the involvement of ipsilateral amygdala (Figure 1, A),
Figure 1 A - Chronic SEEG of a spontaneous complex partial seizure onset in the left hippocampus with involvement of ipsilateral amygdala and persistent epileptic activity in the right hippocampus. 1 and 2 – right amygdala and hippocampus respectively; 3 – right fornix; 4 and 5 - left amygdala and hippocampus. B – a right hippocampal onset of short psychomotor seizure without the involvement of ipsilateral amygdala and less evident continuous interictal epileptic activity in the contralateral hippocampus. 1 and 2 – left amygdala and hippocampus respectively; 3 and 4 - right amygdala and hippocampus.
and without amygdalar participation (Figure 1, B). It is notable that the fornical activity in Figure 1 (A) remained unchanged during continuous epileptiform activity in the right hippocampus, and suggested a relatively lower potential of right hippocampus to trigger a spreading and generalizing seizure. However, the absence of the right fornix participation in this spread suggests the propagation of epileptic discharge through fasciculus uncinatus. Hippocampal and amygdala-hippocampal seizures may develop in both temporal lobes independently, as well as simultaneously with clinical manifestations of psychomotor seizures without convulsive generalization and obvious scalp EEG changes. These different variants of seizure spread were reflected in different EEG and clinical manifestations of seizures observed in the same patient.
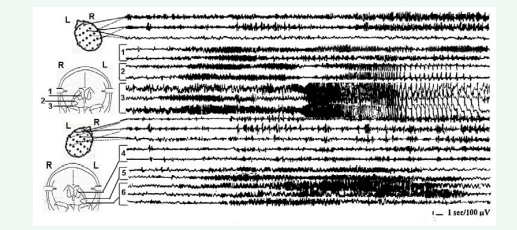
Figure 2 depicts the right focal mesiobasal seizure onset with its spread and generalization presumably through the right fornix and with preferential right cortical involvement.
Figure 2: Intraoperative SEEG recording of the onset and spread of a right mesiobasal seizure, first to ipsilateral fornix with subsequent generalization, and involvement of the symmetrical contralateral mesiobasal structures. 1 and 4 – right and left fornix respectively; 2 and 3 – right amygdala and hippocampus; 5 and 6 – left amygdala and hippocampus.
The involvement of the contaralateral mesiobasal structures developed later. This type of bitemporal epilepsy with secondary generalization primarily through the side of initial seizure onset is an example of when surgery might be limited to unilateral amygdala-hippocampotomy and fornicotomy, despite the involvement of contralateral mesiobasal structures.
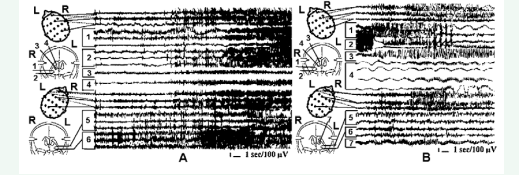
Figure 3 depicts the bilateral spread of a right mesiobasal epileptic seizure with bilateral cingular and cortical involvement and subsequent generalization in a patient with depression and anxiety.
Figure 3: Intraoperative SEEG recording of the spread of a right mesiobasal seizure first bilaterally to the cingulum and scalp EEG with subsequent involvement of the contralateral mesiobasal structures, ipsilateral fornix and generalization. 1 and 2 – left amygdala and hippocampus respectively; 4 and 5 - right amygdala and hippocampus; 3 and 6 – left and right cingulum.
The contralateral amygdala-hippocampal involvement develops after cortical generalization indicating a presumed secondary fronto-temporal seizure spread into the left amygdala hippocampal complex. Additional right hippocampal focal subclinical discharge developed immediately after the cessation of a generalized seizure, emphasizing a heightened epileptogenicity of that structure, and confirmed the need of total hippocampal ablation in this patient. This case could have been also an example of unilateral right amydgala-hippocampectomy, but because of his depression and anxiety, the bilateral amygdalatomy and right hippocampotomy with bilateral cingulotomy was performed. The additional bilateral cingulotomy was performed because of the active cingular participation in the seizure propagation, in addition to severe depression and anxiety in this particular patient.
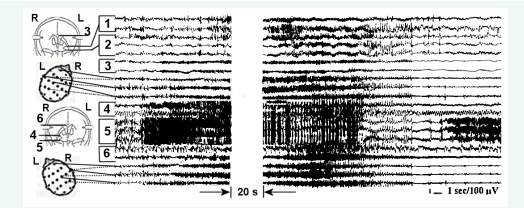
In our cohort of patients, we did not observe initiation of seizures at the diencephalic level. Focal hippocampal seizures may spread to the contralateral hippocampus, and bilaterally over the cortex and generalize without involvement of the anterior thalamic nuclear complex or nucleus Centrum medianum (CM). However, the involvement of thalamic CM nucleus into seizure propagation and generalization may occur through different mechanisms of seizure spread and “maintenance” (Figure 4, A and B).
Figure 4: Chronic SEEGs of a spontaneous secondary generalized complex partial seizure with different variants of thalamic CM nucleus involvement. A – CM is “passively” involved in seizure propagation and generalization. B – CM participates in reverberating thalamo-cortical seizure activity after seizure cessation in the seizure initiating focus. 1 and 2 - right amygdala and hippocampus respectively; 3- right CM; 4- right n. caudatus; 5 and 6-left amygdala and hippocampus
Figure 4 (A) depicts a left hippocampal seizure spreading contralaterally and into the fornix with generalization and continuous involvement of CM and cortex. Part B of Figure 4 pictures a secondary generalized seizure involving CM and continuing in the CM and cortex after the seizure in the initiating epileptic focus in the right amygdala-hippocampal complex had ceased. In the first case (Figure 4, A), CM may play a passive role of just “passing” the seizure through the thalamus, whereas in the second case (Figure 4, B), the non-specific thalamic CM nucleus is included in the thalamo-cortical reverberating circuit synchronizing epileptic activity at this level and maintaining a generalized seizure after the focal seizure initiating discharge had ended.
In patients with epilepsy and concurrent psycho-emotional disturbances, a fast involvement of the thalamic dorso-medial (DM) nucleus and postero-medial hypothalamus (PMH) in their generalized seizures originating from temporal lobe mesiobasal structures was frequently observed. Figure 5 presents the chronic SEEG of a patient with frequent secondary generalized complex partial seizures,
Figure 5: Intraoperative SEEG of a spontaneous secondary generalized complex partial seizure in a patient with interictal emotional instability, fear auras and frequent postictal twilight states with sexual aggression. The spontaneous focal right amygdala-hippocampal seizure propagates into the thalamic DM nucleus before contralateral spread and generalization. 1 and 2 - right amygdala and hippocampus respectively; 3 - right DM; 4 and 5-left amygdala and hippocampus; 6 – left postero-medial hypothalamus (PMH).
interictal emotional instability, fear auras and frequent postictal twilight states with sexual aggression. It is notable that hypothalamo-thalamic entrainment develops prior to the contralateral deep and cortical spread of the initially unilateral deep temporal lobe discharge. This preferential spread of epileptic discharge might cause the specific clinical manifestations in this particular patient. The types of surgeries performed for patients in Group B on the basis of detailed clinico-neurophysiologic analysis of each individual case, and outcomes of these surgeries for seizures are presented in Table 5. For Group B patients, we performed 17 unilateral amygdala-hippocampotomies, 38 bilateral amygdalatomies with unilateral hippocampotomies, 21 bilateral amygdalatomies and unilateral hippocampotomies in combination with contralateral anterior hippocampotomies. The other extratemporal lesions combined with the temporal lobe mesiobasal targets were performed based on the meticulous assessment of all evaluation data.
The 39/76 patients of Class I outcome composed 51% of patients comprising Group B. Worthwhile improvement (Class I-III) was obtained for 60/76 (79%) patients, and no worthwhile Improvement (Class IV) was observed for 16/76 (21%) of patients. Within Class IV results, 9/16 patients had a significant reduction of seizures (Class IV, A). No seizure worsening was observed for this cohort of patients. The relapse of seizures in patients with Class I-III outcomes was observed in seven patients (12%). None of these cases became intractable again.A comparison of outcomes with respect to seizures for Group A and Group B demonstrates considerably better results for Group B, especially in Engel’s Classes I and II (free of seizures and rare seizures) (Table 6).
The neuropsychological assessment of intelligence at the end of hospital stay (approximately two weeks after surgery) demonstrated an initial decrement from baseline. This temporary decrement did not depend on the dominance or non-dominance of cerebral hemisphere and the number and extent of lesions. Full scale IQ scores were almost equally decreased by 5-7 points two weeks after surgery for the both groups of patients. After this postsurgical period, IQ scores for Group A patients very quickly returned to baseline. For the patients of Group B, this period of rehabilitation was delayed up to four-six months and developed even slower for patients with lower presurgical IQ scores. No remarkable further postsurgical improvement was observed for Group A patients at one and more years after surgery, whereas the increase in full scale IQ for 6-9 points was revealed for the Group B patients after six-eight months of surgery. This improvement was more evident in the patients with preoperative scores higher than 85. Unilateral hippocampal lesions were performed in 55 patients. Seventeen of these 55 were associated with ipsi- and 38/55 with bilateral amygdalatomies. One-sided hippocampotomy associated with the partial anterior hippocampotomy combined with bilateral amygdalatomy was performed in 21 cases. Subtle changes of formal neuropsychological tests of naming were found for patients with amygdala-hippocampotomy in the dominant hemisphere and were not observed in patients with the left partial anterior hippocampotomy. These changes were more evident in patients with remarkable preoperative language impairment. We did not observe a postoperative decrease of verbal scores after the right amygdala-hippocampotomy and left anterior hippocampotomy, as well as, no decrease of performance scores after the left amygdala-hippocampotomy and right anterior hippocampotomy. Moreover, there was an increase of the appropriate scores, which probably may be attributed to the hemisphere received a surgery limited by volume (anterior hippocampotomy), but eliminating abnormal seizure activity.
Almost total hippocampotomy in one hemisphere and anterior hippocampotomy in another did not lead to profound memory impairment or additional memory problems in our study. Behaviorally evident short-term memory deficit after such bitemporal interventions was observed in four patients for a few days after surgery, leaving the long-term memory unaffected. Patients could not recollect some events, actions, and conversation immediately proceeding the time of testing. These events lasted for 5-7 days after surgery and disappeared abruptly. Mild recent memory deficit compared to the presurgical state were detectable with memory testing for 2-6 months after surgery for 7/21 patients and did not influenced the patient’s quality of life. These postsurgical memory declines were quickly reversible in the youngest patients (3/17 6-11 y. o. patients in 6-16 years range). We did not find the substantial difference in short- or long-term memory changes in patients with unilateral amygdala-hippocampotomies and bilateral amygdalatomies combined with unilateral hippocampal lesions.
The most remarkable normalization of the psycho-emotional state and behavioral abnormalities was observed in seizure-free (Engel’s Class I, A) and early postoperative seizure (Engel’s Class I, B) patients. This improvement was observed almost immediately after surgery during the postoperative hospital stay and remained stable during the follow-up period. Psychotropic medication for these patients was quickly lowered and withdrawn. In patients, who demonstrated seizures reduction by more than for 75% and continue to have considerably less severe seizures the improvement in the psycho-emotional state was evident, but not as remarkable as in seizure-free patients. Behavioral abnormalities in this group of patients became much milder, and these patients demonstrated better psychosocial adjustment. The psychotropic regimen for these patients was significantly lowered, along with their clinical improvement. Patients who improved with respect to seizures by less than a 75% reduction in seizure frequency and failed to have modified seizure activity showed no clinically evident improvements in behavioral or emotional adjustment.
The complete or almost complete psycho-emotional normalization was obtained in patients with interictal chronic depression and anxiety who received amygdalatomies in combination with cingulotomy. The best results were observed with bilateral lesions and in patients, whose presurgical expectations met the outcome in respect of seizures. The effect of surgery was clearly detectable in 2-3 weeks after surgery, and stabilization was usually observed in 6-8 months. The ictal fear, anger attacks, interictal and “preictal” mood changes, irritability, explosiveness and anxiety were better corrected with bilateral amygdalatomies in combination with postero-medial hypothalamotomy and dorso-medial thalamotomy. The remarkable normalization and stabilization of their psycho-emotional state was usually observed immediately after surgery with stabilization in 3-5 months after surgery with some individual differences, depending on the severity of preoperative symptoms, age of patients and surgery success. The histories obtained from the patients’ families and the authors’ observations during postoperative neurological examinations and EEG evaluations demonstrated that none of the patients showed discernible additional postsurgical deterioration of speech, memory, cognition or behavior.
The scalp EEG dynamics generally followed the course of improvement for seizures. The normalization of postsurgical EEG after the stabilization of the clinical state of the patients of GroupA, was observed in 2/4 Class I and in 2/6 Class II cases. Compared to the preoperative EEGs, no remarkable positive EEG dynamics were observed for the remaining Class I and II patients and for all patients of Classes III and IV. For the patients of Group B, the positive dynamics of postsurgical EEGs were more impressive. The EEG normalization of background activity, disappearance of focal abnormalities, interhemispheric EEG asymmetries, and discontinuation of disseminated sharp activity were observed for 35/44 Class I, 7/11 Class II, and 2/11 Class III (A ) patients. Remarkable improvement first in different degrees of normalization of background activity and reducing of sharp focal and diffuse abnormalities were observed for 6/11 Class III and IV patients with no changes in the remaining five. No postoperative EEG worsening was observed during repetitive EEG evaluations. The EEG improvement followed the clinical improvement closely in the patients with preoperative sharp activity overlapping the normal background. The process of EEG normalization in patients with initially abnormal background heralding a focal or diffuse encephalopathy developed slowly with advanced clinical improvement. For 7/10 patients with the presurgical EEG phenomenon of “forced normalization,” the postsurgical evaluations revealed the disappearance of this phenomenon along with clinical and EEG improvement.
None of our patients had a worsening of their seizures, psycho-emotional state or behavioral abnormalities after surgery. Previously intractable patients with outcome Classes III and IV became more amenable to medication. No persistent life-threatening complications were observed. Surgical complications included one acute subdural hematoma (10 -15 ml) evacuated during the same surgical session through the burr-hole, one minor thalamic hemorrhage with mild left-sided hemiparesis, which completely resolved in two weeks of intensive care, and three cases of subcutaneous infection successfully treated with antibiotics.
Table 4: Types of surgeries performed for Group A patients and their outcomes with respect to seizures (follow-up 1-5 years).
| Types of Surgery | Side of surgery | Outcomes of surgery | ||||||||||||||
| R | L | Class I | Class II | Class III | Class IV | |||||||||||
| A | B | C | D | A | B | C | D | A | B | A | B | C | ||||
| 1. Unilateral VL-thalamotomy | 2 | 1 | - | - | - | - | - | - | - | - | - | - | - | 3 | - | |
| 2. Unilateral amygdalatomy | 1 | 3 | - | - | - | - | - | - | - | - | - | - | 1 | 3 | - | |
| 3. Bilateral amygdalatomy | 6 | 6 | - | - | - | - | 1 | 2 | 1 | - | - | - | 1 | 1 | - | |
| 4. Consecutive unilateral amygdalatomy + ant. hippocampotomy* |
1 | 2 | - | - | - | - | - | - | - | - | - | 2 | 1 | - | ||
| 5. Unilateral amygdalatomy + hippocampotomy |
7 | 3 | - | - | 1 | - | - | 2 | - | - | 4 | - | 3 | - | ||
| 6. Unilateral amygdalatomy + ant. hippocampotomy** |
3 | 2 | 2 | 1 | - | - | - | - | - | - | 2 | - | - | - | ||
| Total | lesions | 20 | 17 | |||||||||||||
| patients | 2 | 1 | 1 | 1 | 4 | 1 | - | 6 | 2 | 6 | 7 | - | ||||
* The interval between consecutive unilateral surgeries was 8 months.
** These five patients received stereotactic amygdale-hippocampal surgery after an unsuccessful anterior temporal lobectomy and postsurgical activation of the contralateral epileptic focus. The interval between consecutive surgeries was approximately one year.
Table 5: Types of surgeries performed for Group B patients and their outcomes with respect to seizures (follow-up 1-5 years). Numbers in parentheses represent the Group A patients received reoperation.
| 0 | Side of surgery¹ | Results of surgery | ||||||||||||||
| R | L | Class I | Class II | Class III | Class IV | |||||||||||
| A | B | C | D | A | B | C | D | A* | B | A | B | C | ||||
| 1. Bilateral amygdalatomy + hippocampotomy |
7 4 |
7 3 |
1(2) | - | 3 | - | - | - | - | 1 | - | - | - | - | ||
| 2. Bilateral amygdalatomy + hippocampotomy + fornicotomy |
12 8 3 |
12 4 9 |
2(1) | 2(1) | - | - | 1 | - | - | - | 2 | - | 2 | 1 | - | |
| 3. Bilateral amygdalatomy + hippocampotomy + bilateral Forel-H-tomy |
5 2 5 |
5 3 5 |
- | 2 | 1 | - | - | - | - | - | - | - | 1 | 1 | - | |
| 4. Bilateral amygdalatomy + hippocampotomy + fornicotomy + Forel’s H-tomy |
8 5 - 4 |
8 3 4 1 |
1 | - | 2(3) | - | - | - | - | - | 2 | - | - | - | ||
| 5. Bilateral amygdalatomy + hippocampotomy + cingulotomy + fasc. uncinatotomy |
6 4 4 1 |
6 2 2 4 |
- | - | 1 | - | 2 | - | - | - | 1 | - | 1 | 1 | - | |
| 6. Bilateral amygdalatony + hippocampotomy + ant. hippocampotomy + bilateral cingulotomy** |
5 2 5 |
5 3 5 |
- | - | 1 | - | 1 | 1 | 1 | - | - | - | - | 1 | - | |
| 7. Bilateral amygdalatomy + hippocampotomy + ant. hippocampotomy + DM-thalamotomy |
9 1 |
9 1 |
2(1) | - | 1(2) | - | - | 1 | - | (1) | - | - | 1 | - | - | |
| 8. Bilateral amygdalatomy + hippocampotomy + ant. hippocampotomy + CM-thalamotomy |
7 5 |
7 2 |
2(1) | 1 | - | - | - | 1 | - | - | - | - | 1 | 1 | - | |
| 9. Unilateral AHT*** + CM-thalamotomy + fornicotomy |
4 2 1 |
2 3 5 |
1(1) | - | - | - | 1 | - | - | - | 1 | 1 | 1 | - | ||
| 10. Unilateral AHT + CM-thalamotomy + Forel-H-tomy |
5 4 4 |
3 2 3- |
1 | - | 1(1) | - | - | - | 2 | - | - | - | 2 | 1 | - | |
| 11. Unilateral AHT + DM-thalamotomy + PMH**** + fasc. uncinatotomy |
1 1 - 2 |
2 2 3 1 |
- | 1 | - | - | 1 | - | - | - | - | - | - | 1 | - | |
| Total patients | 10(6) | 6(1) | 10(6) | - | 6 | 3 | 3 | 1 | 7 | 1 | 9 | 7 | - | |||
¹The numbers in these columns represent number of lesioned structure, not the number of patients.
* Worthwhile improvement means 50 -75% reduction of seizure frequency.
** Cingulotomy means anterior cingular cortex and cingular bundle lesion.
*** AHT stands for ipsilateral amygdalatomy and subtotal hippocampotomy
**** PMH means postero-medial hypothalamotomy.
Table 6: The comparison of the surgery outcomes with respect to seizures for patients of Croups A and B (follow-up 1-5 years)
| Classes of outcome* | Group A | Group B |
| Class I Free of disabling seizures** | 4 (13%) | 39 (51%) |
| Class II Rare disabling seizures*** | 6 (19%) | 13 (17%) |
| Class III Worthwhile improvement**** | 8 (26%) | 8 (11%) |
| Class IV No worthwhile improvement | 13 (42%) | 16 (21%) |
| Total | 31 (100%) | 76 (100%) |
*According Engel et al. (1993).
** Excluding early postoperative seizures.
*** Almost seizure free.
**** 50-75% of seizure frequency reduction.
DISCUSSION
Epileptic focus and epileptic system
A large multicenter study [33] concluded that 77% of intractable epilepsy patients demonstrated 77% of success after mesial temporal lobe resections with a minimal effect on anxiety and depression. Seizures relapsed in 24% of temporal lobe resective epilepsy surgeries. Hennessy et al. [34] found that 35% of seizure relapses came from the contralateral hemisphere and 30% from the contralateral temporal region. These data demonstrate how frequently active elements of epileptic systems remain undetected, and hence persist even with contemporary technically advanced presurgical evaluation. In addition, we have to keep in mind the 30% of intractable epilepsy patients who were not considered for surgery because of multifocality of seizures, localization of epileptic focus (foci) within eloquent cortical areas, or possible postsurgical memory impairment.
The present indications for epilepsy surgery are based on the conception of a single epileptic focus generating the seizure, followed by seizure propagation and involvement of other brain structures. It is suggested that surgical removal of that epileptic focus should make patient seizure free. However, clinical experience and practice demonstrated multifocality of seizures in patients with intractable epilepsy and frequent relapse of seizures after such limited surgeries. This forced the surgeons to expand their surgical tactics, and perform combined resections, or multiple stereotactic lesions. Multiple lesions seemed to be necessary for the better control of epilepsy [3,35-39]. Analysis of the literature demonstrates that even conventional resective multilobar and bihemispheric epilepsy surgery [40], combinations of topectomies with multiple subpial transections on both hemispheres, callosotomies and stereotactic amygdala-hippocampotomies [41-44], and multiple cortical thermolesions [45] can be performed without neurological and neuro-psychological complications. Zemskaia et al. [46] performed bilateral one-stage stereotactic interventions on mesiobasal temporal structures or stereotactic operation on one temporal lobe and an open operation on the contralateral temporal lobe in patients with bitemporal epilepsy. These data suggest that the existing conception of an epileptic focus, especially in cases of severe intractable epilepsy, needs additional elaboration.
The concept of an epileptic focus was revised. The difficulty of identifying the precise location of brain structures initiating epileptic seizures has led some authors away from the concept of a strictly localized epileptic focus. A concept of “regional epilepsy” was conceived, which in the case of temporal lobe epilepsy, included orbital, temporal and anterior cingulate areas [47]. The author suggested that the concept of focal epilepsy being related to focal (partial) seizures through one epileptic focus or cortical area is an “overschematized simplicity“ and tended to de-emphasize the true complexity of disease and our fragmentary knowledge of the pathophysiology of epilepsy. Collins & Caston [48] concluded that the symptoms of focal epilepsy are not the expression of a single focus, but rather the expression of its associated “circuits.” According to Engel [4,49], in cases of intractable epilepsy the brain of the epileptic patient “appears to be abnormal in many different areas and in many different ways. ” So et al. [7] found that epileptic seizures arising from the same temporal lobe in the same patient could start independently in larger or smaller areas within a wide epileptogenic zone. Although many authors have articulated the coexistence of discrete epileptic foci in different brain areas, they have not presented the idea of a dynamically organized functional entity or system.
Epilepsy, especially intractable epilepsy, may be considered as a dynamic multifactoral process including alteration in neurotransmitter receptors and synaptical plasticity, ion channelopathies, and reactive autoimmunity [4,5,8-13]. This leads to the reorganization of neuronal circuitry and formation of a complex and individually organized epileptic system, including dominant and subdominant epileptic foci and seizure propagating pathways. Chronic and/or intraoperative depth electrode studies have demonstrated the complexity and multistructural organization of epileptic networks in intractable epilepsy patients [7,13,30,50-58]. Wiser [2,53] and Spencer [13] systematized the results of their studies, subclassified complex partial seizures into several subtypes, and described more or less typical variants of a “cast” of structures participating in the spread and generalization of seizures originating in the temporal lobe mesiobasal structures. It was hypothesized that the epileptogenic circuit for the initiation of seizures is distributed throughout the limbic system with a possible central synchronizing process [8]. Based on this concept, the limbic epilepsy surgery failures were attributed to incomplete resections in seizure circles and more extensive resection of limbic structures with defined contributions from the contralateral limbic system was suggested [59]. Most of the authors described the interrelations of brain structures and seizure propagation variants in general, not in relation to the particular patient to whom these variants were responsible for individual diversity of illness and without a recommendation of individual surgical tactics.
All these data allow us to view severe long-standing intractable temporal lobe epilepsy not as just focal epilepsy, but as focal epilepsy with a dynamically and individually organized epileptic system [3,11]. The concept of a single epileptic focus generating seizure followed by seizure propagation and involvement the other brain structures should be conceptualized as dominant and subdominant or dormant epileptic foci, and a network including not only pathways and structures involved in the spreading seizure, but actively participating in the epileptic process. Such insight on the problem of surgical treatment of severe long-standing intractable temporal lobe epilepsy dictates a comprehensive evaluation of patients in order to determine the interrelations between the epileptic system core elements and performing an optimal neurophysiologically guided surgical procedure for each patient.
Interictal and ictal activity of the epileptic system
The main limiting factor of our study is an inability to have electrodes implanted in all brain structures. We tried to, in some degree, to avoid this factor by a meticulous pre-implantation analysis of the patients’ neurological status, seizure manifestations, peculiarities of these manifestations and seizure generalization, and neuro-psychological and imaging data. The analysis of deep temporal lobe electrical activity in both of our groups of long-standing intractable epilepsy patients revealed bilateral involvement of temporal lobe mesiobasal structures in the epileptic process practically in all patients. These data are consistent with results of an SEEG study of another group of our patients [28] where bilateral involvement of temporal lobe mesiobasal structures was found in 66% of patients. This raises the question of whether such bilateral amygdala-hippocampal involvement is typical for long-standing intractable epilepsy patients, and if it serves, along with other factors (multidrug resistance-associated protein, proteins associated with drug resistance in cancer, major vault protein), as a neurophysiologic basis of epilepsy intractability.
The existence of bilateral independent or propagated epileptic activity was reported at the beginning of the depth electrode era [60-62]. The role of the commissural system and pathways of seizure interhemispheric spread were discussed by many authors [6,20,54,63-66]. Clinical investigations in patients with multicontact electrodes revealed strong evidences that seizure discharges originating in the deep structures of one temporal lobe can spread to contralateral structures without prior involvement of thalamic nuclei or ipsi- and contralateral neocortex [6,36,53]. The important role of orbito-frontal cortex in the interhemispheric propagation of temporal lobe seizures was also demonstrated [55,67]. All of these data indicate that the interaction of brain structures composing an epileptic system may be realized through multiple pathways.
The participation of thalamic nuclei in human epilepsy has been discussed for long time [68-70], more recently with attempts to treat epilepsy with direct brain stimulation [71-77]. In our cohort of patients we, as well as Wieser [54], did not observe an initiation of seizures in thalamic structures, but often recorded thalamic nuclei participation in the propagation of seizures (Figure 4, A) or in the “synchronization” and maintenance of seizure activity in a thalamo-cortical reverberating circle, even after initiating mesiobasal focal activity has ceased (Figure 4, B). This participation of thalamic midline nuclei in the propagation of epileptic seizures is supported by the latest experimental data [78]. A cortico-thalamic coupling of metabolism revealed using the fMRI data, probably detected such variants of thalamic participation in the epileptic process [79].
Varieties of surgery and indications for specific types of surgery.
All of our surgeries were guided by meticulous analysis of neurophysiologic data obtained during the pre- and intraoperative evaluation of patients. The surgical interventions on the amygdala-hippocampal complexes were considered as “core” surgery, and the lesioning of other brain structures was dictated by the specific clinical, neuropsychological, and electrophysiological peculiarities of each of case. As mentioned above, an apparent unilateral epileptic focus was found in 17% (16/93) of cases. For the remaining 77 patients, bilateral interictal and ictal epileptic activity was assessed as predominantly unilateral in 18 cases (19% of all 93 patients). Unilateral surgeries were performed in all 31 patients of Group A (surgery types 1-6) and 17 patients of Group B (surgery types 9-11). During amygdala-hippocampotomies, we usually tried to perform a total or subtotal lesion of these structures, keeping in mind that small amygdalar lesions might be insufficient to control seizures [80]. This opinion was later supported by comparison of outcomes of stereotactic amygdala-hippocampotomy in one group of patients with lesions encompassing amygdala and 13-21mm (mean 16.8 mm) of anterior hippocampus, with another group of patients to whom anterior hippocampal lesion was extended to15-34 mm (mean 21.5 mm) [81]. The difference just of 4.7 mm gave a threefold increase in favorable results. The therapeutic effect of amygdalatomy is not only the lesion of an epileptogenic tissue and normalization of psycho-emotional state and behavior, but also prevents the spread of seizure discharges from the amygdala-hippocampal complex to the frontal lobe through the fasciculus uncinatus [82]. This may explain, in part, the success of amygdalatomy against epileptic seizures in some cases when the hippocampus was left intact [83,84]. The second important peculiarity is that homolateral amygdala and hippocampus are practically always involved together in epileptogenesis. The hippocampus was considered as a core part of the “medial emotional circle” [85]. Later, the “baso-lateral emotional circle” was described with the amygdala as its important part [86]. In epilepsy, besides seizure generation, the combined abnormal functioning of these two structures is responsible for psycho-emotional and behavioral abnormalities, and makes both of these structures important double targets for the treatment of intractable epilepsy patients with psycho-emotional and behavioral disturbances.
In the patients with interictal, preictal, and postictal psycho-emotional disturbances, the thalamic, hypothalamic, and limbic cortical structures are consistently involved in the epileptic process. Recent studies found that postictal psychoses in partial epilepsy are associated with broadly and bitemporally distributed epileptogenic network [87]. Our previous investigations with chronically implanted electrodes demonstrated a direct interrelation between amygdalar and hippocampal activity and exacerbation of psycho-emotional abnormalities in epileptic patients [3,88]. It was concluded that ictal fear is related to pathology of the amygdala and that it, like the hippocampus, is an important substrate of temporal lobe epilepsy [89]. Later, metabolic changes were described in the head of the hippocampus in patients with ictal fear [90]. Cingulate participation in partial epilepsy was reported earlier [91,92]. We found that cingulate involvement in the process of seizure generalization was frequently observed in patients with psycho-emotional disturbances, especially with depression and anxiety as a major complaint confirmed with neuro-psychological testing. This cingulate involvement was usually characterized by rapid contralateral cingular spread and subsequent spread to the frontal cortex. Thalamic dorso-medial nucleus (DM) and postero-medial hypothalamus are frequently involved in the seizure spread in patients with interictal, preictal fear and rage attacks, postictal twilight states and hypersexual behavior.
The difference between Group B patients who underwent unilateral surgery is that in addition to amygdala-hippocampotomy, cryo-lesions in CM and fornix (type 9 surgery), CM and Forel-H-field (type of surgery 10), and DM, PMH, and fasciculus uncinatus (type of surgery 11) were performed. CM lesions were performed because of SEEG verified participation of this nucleus in the propagation and synchronization of seizure activity (Figure 4). Fornicotomy was performed because of frequent secondary generalization of seizures and SEEG-verification of fornical involvement (Figure 2). Forel-H-tomy was performed because of fast secondary seizure generalization after spread over ipsilateral frontal cortex and fornix preceding contralateral involvement. DM and postero-medial hypothalamic lesions were performed on patients with major psycho-emotional disturbances and SEEG verification of the involvement of these structures in the epileptic process. Fasciculus uncinatus lesions were performed because of fast clinical generalization of unilateral focal seizures and predominant involvement of homolateral fronto-temporal areas in the seizure spread (Figure 5).
The same criteria of choosing additional targets inside the epileptic system were used during bilateral surgeries with some additional peculiarities. Bilateral amygdalotomy was performed for all 59 bilateral surgery patients of Group B (surgery types 1-8). The indications for bilateral amygdalatomy were a high level of interictal epileptic activity in both amygdalae without obvious prevalence, participation in subclinical and clinical seizures developing in both temporal lobes, and, in most cases, evident psycho-emotional disturbances. For the 21 patients of Group B, we performed total hippocampotomy on one side and partial anterior hippocampotomy on the contralateral side (surgery types 6-8). The criteria to perform these asymmetric surgeries on both hippocampi were apparent bitemporal independent EEG/SEEG onset of seizures in both hippocampi, the distinctive manifestations of the clinical seizures, and mutually suppressive interactions of hippocampal epileptic foci, heralding possible activation of another hippocampal epileptic focus after the ablation of one of them [28,31]. Before performing full-size partial anterior hippocampotomy, we undertook an additional study of 10 similar patients (not included in this series) with small control electrolytic anterior hippocampal lesions ranging in diameter from 2 to 8 mm. Postsurgical neuro-psychological testing did not reveal additional memory deficits, compared with their preoperative state.
Surgery outcomes regarding the seizures and psychoemotional abnormalities
A relapse of seizures in patients with Class I-III outcomes was observed in 7 patients (12%). The relapse of seizures during 1-5 years of follow-up is higher than that recently reported (4%) after temporal lobe resective surgery [93], but there is a considerable difference between the groups of patients and indications for surgery. These results, comparable to resective temporal lobe epilepsy surgery results, are obtained with patients who usually remain beyond the scope of indications for surgery and do not expect any help.
The comparison of outcomes with respect to seizures in Group A and Group B (Table 3) demonstrates considerably better results for group B, especially for Engel’s Classes I and II (free of seizures and rare seizures). These data indicate that the efficacy of multitarget lesioning of the key elements of the epileptic system is comparable (Table 6) with the 46% to 78% of successful results of temporal lobectomy in patients with strongly localized unilateral temporal lobe epileptic foci [14,33,94,95].
The main obstacle and concern with epilepsy surgery of patients with poorly localized or bitemporal epileptic foci, suggesting a multifocality of seizures, psycho-emotional and psycho-social problems, are a dread of such surgery complications as memory and personality impairment. This fear stems from Klüver & Bucy’s [96] findings, which demonstrated that bilateral resection of temporal lobes including temporal lobe cortex, hippocampus, and amygdala produces a “psychic blindness” syndrome in monkeys. Later, Scoville [97], and Scoville & Milner [98] described recent memory loss after bilateral hippocampal lesions. A review of these cases did not reveal a precise surgery limited with hippocampal ablations, but rather extensive bilateral resection of the medial surface which extended 8 cm posteriorly from the tip of temporal lobe, performed through Scoville’s bilateral fronto-orbital approach. Terzian & Ore [99] described bilateral temporal lobe resections both extended up to the vein of Labbe in a patient with bilateral independent EEG epileptic foci who exhibited some elements of Klüver-Bucy syndrome associated with severe memory loss. Apparently, the volumes of these surgeries, number and extend of bilaterally resected temporal lobe structures including lateral, basal cortex, hippocampal, parahippocampal gyri and entorinal cortex are not comparable with precise and controllable stereotactic lesions, which do not include the whole extent of both hippocampi. The dependence of the degree of cognitive, learning, and memory functions on the degree of surgical intervention and surgical approach was also reported by Wieser & Yasargil [100], who found less or even no impairment after selective amygdala-hippocampectomy compared to anterior temporal lobectomy. Many authors attribute the memory impairment after experimental or clinical temporal lobes ablation to the different parts of temporal lobe cortex. Ojemann & Dodrill [101] emphasized the importance of temporal lobe lateral cortex for verbal memory. Joo et al. [102] found that the resection of inferior and basal temporal lobe gyri leads to an impairment of verbal memory. Halgren et al. [103] recorded neuronal unit activity in the mesiobasal structures during psychological tests and found that only hippocampal gyrus neurons responded during recent memory recall. The participation of specifically hippocampal gyrus in recent memory mechanisms is confirmed by intact recent memory after bilateral fornicotomy [104,105] and with disrupting memory with cingulum stimulation [106].
The almost total hippocampotomy in one hemisphere and anterior hippocampotomy in another without any additional lesions in temporal lobe cortex, especially the hippocampal gyrus, did not lead to profound memory impairment or additional memory problems in our study. Behaviorally evident short-term memory deficit after such bitemporal interventions was observed in four patients a few days after surgery, leaving long-term memory unaffected. We did not find a substantial difference in short- or long-term memory changes in patients with unilateral amygdala-hippocampotomies and bilateral amygdalatomies combined with unilateral hippocampal lesions. The elucidation of mild or moderate postsurgical memory changes in the most of our patients was probably impeded because of their presurgically impaired memory. Such subtle postsurgical memory changes might be explained with continuous or intermitted discharges in the amygdala- hippocampal complex already functionally “resected” these structures, and their real surgical ablation did not add a further deficit. We did not observe a postoperative decrease of verbal scores after right amygdala-hippocampotomy and left anterior hippocampotomy, as well as no decrease of performance scores after left amygdala-hippocampotomy and right anterior hippocampotomy. Moreover, there was an increase of these scores of a few points, probably because of an absence or decrease of a disturbing influence of intermitted or constant epileptic activity in the contralateral epileptic focus. The amelioration and return to normal social life and in some cases even rise in IQ for epileptic patients after bilateral amygdalatomy and unilateral hippocampotomy have been reported [107,108].
Persistant abnormal activity in mesiobasal temporal lobe structures has the same disturbing effect on cognitive, learning, and memory function as their ablation. Transient retrograde amnesia was also observed after widespread disruption of the mesial temporal lobe by electric stimulation [109,110] . It is found that subclinical discharges may be associated with transitory cognitive impairment detectable by appropriate psychological testing [111] In epilepsy patients with implanted depth electrodes, it was found that fast spiking in the hippocampus might be responsible for the memory deficits in patients with epilepsy [112]. These data support the hypothesis that subclinical epileptic activity in the hippocampus disables its normal functioning and may simulate its “functional ablation. ” The absence of substantial difference in short- or long-term memory changes in patients with unilateral amygdala-hippocampotomies and bilateral amygdalatomies combined with unilateral hippocampal lesions suggests limited amygdala participation in the processes of memory. We already reported successful stereotactic amygdalatomy in 8/14 bitemporal epilepsy patients who developed an activation of the contralateral epileptic foci after temporal lobotomy [28]. These results are supported by data that even large bilateral amygdala lesions fail to affect learning or retention of verbal materials [113].
Seizure-free patients achieved significant and stable improvements in behavioral and emotional adjustment approximately six months after surgery, whereas in patients with less favorable outcomes for seizures this adjustment was less evident and stabilized at lower level in eight months to one year. In 10 patients with presurgical anger attacks, aggression, periodic psychotic states, and EEG phenomenon of “forced normalization” [114], postsurgical evaluations revealed the disappearance of this phenomenon for seven patients, along with clinical and EEG improvement. SEEG evaluations revealed a high level of interictal and ictal epileptic activity in the amygdala with involvement of the posterior hypothalamus thalamic dorso-medial nucleus. Our previous studies performed with chronically implanted deep electrodes demonstrated that despite the “normalization” of the scalp EEG, anger attacks, destructive behavior, and sexual aggression are consistent with increased intermittent epileptic activity and “subclinical” epileptic seizures in temporo-limbic structures [115]. These findings are important in terms of clinical, EEG, and behavioral assessment of the results of surgery. For patients who exhibited a reduction or complete cessation of convulsive or psychomotor seizures after surgery with evident EEG improvement, but demonstrate unchanged or increased psycho-emotional and behavioral disturbances, it is necessary to be careful with the final assessment of surgery outcome. This group of patients represents a “group of risks,” and relapse of clinical seizures in this group may be more likely.
CONCLUSION
Our results demonstrate that multitarget electrophysiologically guided stereotactic surgery can have a beneficiary effect on seizure frequency and severity, normalize psycho-emotional state and behavior in long-standing intractable epilepsy patients who, in most cases are not considered as optimal candidates for resective epilepsy surgery. Correctly and carefully planed multitarget stereotactic surgery does not necessarily lead to additional and stable postoperative declinies in intelligence, learning, and especially memory, and the benefits of seizure control definitely outweigh the risk of further cognitive decline. Moreover, according to the extent of surgery and results obtained, this tactic can be considered as a minimally invasive approach to intractable epilepsy surgery. This article does not intent to replace resective epilepsy surgery when it can be highly beneficial. The aim of this study is to advocate the resurgence of electrophysiologically guided stereotactic lesional epilepsy surgery, based on practically applied existing knowledge about sophisticated epileptic systems in cases of severe intractable epilepsy, as well as, the implementation of more effective lesional methods. This approach to epilepsy surgery may include different reasonable combinations of resective, stereotactic lesional, stimulation and cortical transection techniques directed toward beneficiary treatment of these intractable epilepsy patients.
ACKNOWLEDGMENTS
The authors thank Dr. Vernon L. Towle, Ph. D., for very helpful comments and help in manuscript preparation.