Multiple Sclerosis Treatment Update
- 1. Department of Neurology, Oklahoma University, USA
- 2. Oklahoma City VA Medical Center, USA
Abstract
In the past 20 years there have been many notable advances in the treatment of multiple sclerosis (MS). This review provides an overview of the recent advances which have taken place in management of patients with MS, rather than just discussing in detail the findings of various randomized trials of recently approved and/or in process of being approved.
Keywords
• Multiple sclerosis
• Immuno-Modulatory therapies
• Oral therapies
• Neuroprotection
• Remyelination
• MS-related disabilities
Citation
Rabadi MH (2014) Multiple Sclerosis Treatment Update. J Neurol Transl Neurosci 2(2): 1047.
INTRODUCTION
Multiple Sclerosis (MS) is a chronic inflammatory disease of the central nervous system, with a typical onset between the ages of 20 and 40 years, and women are affected more frequently than men in a ratio of 2.5:1. It has a variable clinical presentation and course. In 70% to 80% of the patients begin with a relapsing-remitting course (RR) [1]. A relapse is considered an attack or exacerbation where there is a recurrence of the old or new symptoms developing over days to weeks, accompanied by objective findings on a neurological examination. Most relapses recover partially or completely over weeks to months even in the absence of treatment. In ~ 15% cases patients begin with a progressive course where symptoms persist overtime (PP). Patients with PP MS tend to be older with less female predominance. MS is the leading cause of disability in the young due either to incomplete relapse recovery [2] or to the progressive nature of the disease. It is difficult to prognosticate in individual cases early in the disease as to its clinical course. In 10% to 20% of cases MS may have an indolent course with minimal disability over time, while in 5% cases may have a fulminant course with frequent relapses and rapid progression of disability. Factors suggestive of a favorable prognosis include female sex, mainly sensory symptoms, RR course, infrequent or mild relapses with good recovery and a low MRI lesion load (T1-hypointense and T2-hyperintense lesions) and atrophy [3,4].
PATHOGENESIS
Research has shown MS to be a T-cell mediated inflammatory, demyelinationg disorder of the central nervous system. While destruction of myelin sheath is considered to be T-cell medicated, there is recent evidence to support B-cell involvement and humoral immune mechanisms including intrathecal antibody production, autoreactive antibody in cerebrospinal fluid, complement deposition with myelin disruption in MS lesions, and the presence of B-cells in perivascular cuffs and meningeal lymphoid follicles [5] adjacent to the cortical structures responsible for the relapsing-remitting and the progressively worsening features of MS. Exposed axons from myelin sheath destruction result in alteration of voltage-gated Na and K ion channels and its transporters causing intracellular calcium accumulation leading to eventual neuronal cell death [6].
IMMUNOMODULATORY THERAPIES
Since the 1990’s ten immunomodulatory therapies (IMTs) (also called Disease-Modifying Therapies) have been approved for the treatment of RR MS. The current IMTs are largely anti-inflammatory in nature focused in mediating T-cell response. The main-stay medications have been the first-generation injectable “A, B, C’s”, interferons-α and β (Avonex, Rebif, and Betaseron) and glatiramer acetate (Copaxone) [7-9]. They are usually safe, modestly effective (30% reduction in relapse rate and a decrease in MRI T2 lesion burden and gadolinum enhanced lesions), a nuisance for the need to self-inject, and a range of side effects such as injection site reaction, flu-like symptoms, or depression, all resulting in a high rate of noncompliance [10]. There are patients who, despite being compliant on these medication face frequent clinical relapses or increasing plaque burden on brain MRI [11]. It has been shown that patients with relapsing-remitting MS (RRMS) on first-generation IMTs are more likely to switch therapies if they present with MS at a younger age, perceive lack of efficacy, and have relapses during the initial 6 months of their IMT treatment [12]. Patients with treatment gaps of 90 days or longer are twice as likely to experience a severe MS relapse as patients with shorter treatment gaps [13]. When switched to newer medications their benefits out-weigh the risk, the potential for serious side-effects. The new medications include monoclonal antibodies such as Natalizumab (Tysabri) [14-16] and recently Alemtuzumab awaiting FDA approval [17- 19] and oral medications such as Fingolimod and Tecfidera. Use of Natalizumab has the advantage of monthly IV infusion and shown to decrease the inflammatory response (frequency of relapses and plaque burden), however; its use has been associated with progressive multifocal leucoencephalopathy (PML). PML is characterized mainly by cognitive impairment due to decrease in innate immune response leading to activation of John Cunningham virus (JCV). It is now a standard of practice to stratify for PML risk [20] (Figure 1)
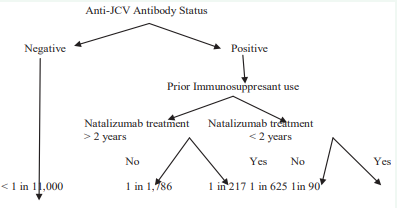
Figure 1 Risk Stratification for Progressive Multifocal Leukoencephalopathy
which includes anti-JCV antibody blood test, and inquiring about prior use of immunosuppresants and/or chemotherapy. Patients also need to be enrolled in the Tysabri Outreach: Unified Commitment to Health (TOUCH) [21] program before starting Natalizumab. Prior to their monthly infusion patients should have a blood count and test for liver functions. Yearly anti-JCV antibodies titers are necessary while taking this drug. Patients suspected of developing PML should stop natalizumab treatment, perform brain MRI and an LP for presence of JCV in the CSF, and undertake plasma exchange (PLEX) to remove natalizumab from the circulation, allowing immune reconstitution of the central nervous system [22]. Removal of the drug can lead to an immune reconstitution inflammatory syndrome (IRIS) which responds to corticosteroids [23].
It is suggested that alentuzumab like natalizumab should be reserved for the treatment of severe and refractory MS. Patients taking alemtuzumab frequently face infusion-related reaction due to cytokine release and need antihistamines, IV infusion of methyprednisolone (steroids), and antipyretics during the initial three days of treatment. They are also at an increased risk of infections frequently involving the upper and lower respiratory tract and urinary tract, and in the second or third year of treatment of commonly developing autoimmune thyroid disorder (hyperthyroidism) and rarely immune thrombocytopenia which requires a close watch for petechial rash, bruising, and bleeding. It is advised that blood and thyroid function tests should be performed on a regular basis and testing should continue for four years after the last dose. The infrequent infusion schedule in alemtuzumab (12 mg administered in 2 annual treatment courses. The first course is given as an intravenous infusion over 5 consecutive days, and the second over 3 days 12 months later) in itself presents a challenge to make sure patients routinely present for their clinic follow-up and for their infusion appointments. Interleukin 21 (IL-21) is being considered as a biomarker for patients on alentuzumab who are at risk for developing its autoimmune adverse effects.
Given the modest efficacy, frequent side-effects, and the need for frequent injections non-compliance is a major issue in a patient with MS. Oral medications such as Fingolimod, a sphingosine-1-phosphate 1 receptor modulator [24,25] and fumaric acid derivative, Tecfidera, an inducer of T-lymphocytes apoptosis [26,27] have recently been approved by the FDA and are increasingly been used. Before starting patient on Fingolimod a structured evaluation is needed which includes, an inquiry if the patient is on any anti-arrhythmic or immunosuppressant? Patient should have an eye examination to exclude retinal and macular diseases as it causes macular edema. The first dose has to be administered in-house with constant telemetry-monitoring as it can cause bradycardia or AV conduction blocks [28]. Both Fingolimod and Tedfidera can cause elevation in the hepatic enzymes (transaminases) and lymphopenia making the patient susceptible to serious infection and liver disease. Consequently blood count and liver function tests are recommended before starting the medication and on regular follow-up. If severe lymphopenia (< 0.5x109 ) occurs on either medication the patient should stop taking the medication and recheck the lymphocyte count after 4 weeks. The medication can be restarted once the count has improved to at least low normal. During this time period patients should not receive any attenuated live vaccine. Initiation of Tecfidera can be associated with flushing, and complain of gastrointestinal upset with nausea, vomiting, diarrhea, and even abdominal pain. These complaints are usually mild and wear off after a few days of use. Patients are advised to take the medication after a full breakfast in the morning and an evening meal and not on an empty stomach as these complains can result in patient’s discontinuing the medication. Patients who become pregnant on this medication should enroll in the Tecfidera pregnancy registry.
Finally of the first-generation IMT’s, copaxone approved as a 20mg daily subcutaneous (s/c) injection [29] has been studied as an alternate day dosing regimen. The advantage of less frequent weekly s/c injection would result in significantly fewer localized injection reactions including lipoatrophy compared to daily injections. The GALA (Glatiramer Acetate Low-frequency Administration) study found GA 40mg s/c thrice weekly to be a safe and effective regimen for the treatment of RRMS, providing the convenience of fewer s/c injections per week while maintaining a similar weekly dose as the approved 20mg regimen [30].
NEUROPROTECTION
The underlying neurodegeneration in MS is due to cell death from increased intracellular calcium accumulation in neuron and glial cells from glutamate excitotoxicity due to ion channel dysfunction. It is now accepted that this is the main cause for MS-related disability [31]. Focus is now on drugs with neuroprotective effect (salvage brain tissue). Of the IMT’s copaxone [32] and a third oral medication laquinimod, a linomide derivative and possible modulator of T-helper cells [33] has been shown to have neuroprotective effect. Laquinimod reduced annualized relapse rate by 20%, brain atrophy by 30%, and reduction in progression of disability by 46%.
Other agents include riluzole and lamotrigine. Riluzole, a kainate and N-methyl-D-aspartate (NMDA) receptor blocker inhibits the glutamate release from nerve terminal and commonly used in patients with amyotrophic lateral sclerosis was tried in 16 patients with primary progressive MS. Results were mixed with riluzole reducing the rate of cervical cord atrophy and the development of T1 hypointense lesions on magnetic resonance imaging but having no effect on the rate of brain atrophy [34]. Possible neuroprotective effect of lamotrigine, a sodium-channel blocker was studied in 120 patients with secondary progressive multiple sclerosis. This was a negative study as after 2-year follow-up the brain volume did not differ from those on placebo; instead patients on lamotrigine had an early brain volume loss that partially reversed on discontinuation of treatment [35].
In view of lack of confirmed neuroprotectants in randomized trials in patients with MS Vitamin D has been shown to have a protective effect on the risk of developing MS [36]. Patients with low levels of vitamin D appear to be at an increased risk for the number of relapses, number of new active lesions on MRI, and the amount of brain atrophy over a 5-year period [37]. The level of serum vitamin D (25-hydroxyvitamin D) considered to be sufficient to maintain bone health with no adverse side-effects is 75 nmol/L or higher [38]. Vitamin D supplementation also has the added benefit of reducing the risk for developing osteoporosis and the increased risk of bone fractures.
REMYELINATION
Attempts are being made to find agents that would help promote axonal remyelination (neural repair) and decrease disability associated with progressive MS. Promoting remyelination with transplantation of oligodendrocyte precursor cells (OPC) has not been helpful [39]. Attempts have been made to stimulate OPC by fingolimod to promote its survival and differentiation into oligodendrocytes [40], enrich trophic environment such as administration of growth factor [41] and neutralize hazardous micro-environment such as lingo-1 [42] and prolactin [43].
MS-related disabilities
Of the MS-related disabilities; fatigue, cognitive impairment and limited ability to ambulate are frequent and can be most disabling. Dalfampridine, a slow release K+ channel blocker, has been shown in randomized clinical trials [44] and in common daily usage to help improve ability to walk in these MS patients and also improve their functional activities of daily living [45]. Cognitive impairment particularly memory dysfunction occurs in approximately half of the individuals with MS and is a leading cause of MS-related disability. It is significantly (albeit weakly) correlated with physical disability [46]. Cholinesterase inhibitors such as Donepezil and Rivastigmine commonly employed in Alzheimer’s disease has been tried in MS patients with mixed results. In the initial single-center placebo controlled trial of 69 participants showed donepezil relative to placebo improved verbal memory, however follow-up larger multi-center trial of 120 participants study on donezepil and recent rivastigmine to placebo in 86 patients with MS showed no treatment effect [47- 49]. Likewise, use of ginko biloba to placebo in 120 patients with MS showed no improvement in cognitive performance in the treatment group [50]. Several cognitive rehabilitation therapies in randomized controlled trials have been found to be effective in improving learning and memory impairment in MS [51-53].
Personal perspective of treating patients with MS
Once the diagnosis has been established based on history, physical examination, brain and spine MRI, and cerebrospinal fluid findings, the general consensus is to start treatment in patients with RR MS and in patients with Clinical Isolated Syndrome (CIS) in the presence of brain MRI lesions as they are at a high risk for developing MS in the future. The choice of selecting an individual IMT is usually based on the discussion between the physician and the patient. We prefer to treat our patients with Glatiramer Acetate (GA) as it has fewer side-effects than Interferons (IFN) with similar efficacy. It is especially helpful in patients with pre-existing depression, spasticity and headaches. Its only drawback is the need for daily injections. In any case, IFN preparations or GA are acceptable options for initial therapy. Recently, we have also started treating our MS patients on oral medications such as Fingolimod and Tecfidera in cases where patients have refused injectable IMTs. Though the FDA recommends patient to be observed for 6 hours following the first dose, we prefer to observe the patient for at least 24 hours following the first dose as some of the patients have experienced bradycardia and AV conduction block more than 6 hours later. Given that compliance is an issue with patients with MS, in our clinic at the VA Medical Center we follow the patients every 4-months and patients are advised to present as a walk-in should they experience major medication side-effects or worsening MS-related disabilites. Every 2-years brain MRI is undertaken if patients are stable. Every year patients on Tysabri are tested for anti JCV antibody titer.
In patients experiencing ongoing disease activity after initiating therapy with standard agents, and in the presence of new brain MRI lesions we may switch to another class of IMT such as IFN from GA, however; lately we have switched them to oral medications for ease of administration and compliance. We do use monthly IV infusion of Natalizumab in patients with fulminant MS, who present with frequent severe relapses that end in incomplete recovery. Some of our patients with secondary progressive MS continue to take their standard IMT which they took for their RR MS as they are fearful of a relapse which may occur. At present for PP MS we have no available IMT to offer, though one should at least obtain a brain and spine MRI to not only delineate active lesions but also rule out a treatable cause responsible for the progressive disability.
Address MS-related symptoms concern such as fatigue which may need amantadine or modafinil, for walking impairment dalfampridine, for neurogenic bladder oxybutynin, for neuropathic pain neurontin, pregabalin, or duloxetine, and for spasticity baclofen, gabapentin or tizanidine. Finally no MS treatment is complete without checking for serum vitamin D levels and if low ( 75 nmol/liter.
CONCLUSION
In conclusion major advances have been made in the treatment of relapsing-remitting MS, at the present time we have been unable to find a cure for the disease neither do we have medications for patients with progressive MS. In the last 25 years we have come a long way in understanding how best to treat MS from administering anti-inflammatory agents (IMT’s) to trying our best to preserve brain tissue (neuroprotection) and even help with neural repair (re-myelination). Readers are advised to read excellent recent reviews of current medications in MS by Brück W et al. and Hauser SL et al [54,55].






































































































































