Paired Associative Stimulation to Suppress Contralesional Corticospinal Excitability in Three People with Stroke Using N-of-1 Crossover Design
- 1. Department of Rehabilitation Science, University of Minnesota, USA
- 2. Institute for Engineering in Medicine, University of Minnesota, USA
- 3. Department of Physical Therapy, University of Minnesota, USA
- 4. Department of Biostatistics, University of Minnesota, USA
Abstract
Background: Some of the deficit in people with stroke results from down-regulation of surviving neurons. Up-regulation can be enhanced with transcranial magnetic stimulation methods. Paired associative stimulation (PAS) is one such method but this has not been explored sufficiently in stroke. Further, N-of-1 clinical trials are valuable in eliminating inter-subject variability to develop individualized interventions.
Objective: Explore the effects of PAS using different interstimulus intervals (ISIs) to suppress excitability of the contralesional primary motor area (M1) in stroke.
Methods: We used an N-of-1, blinded, crossover design with random assignment of four PAS treatment arms to three people with stroke. Each PAS treatment involved 225 pairs of transcranial magnetic stimuli to contralesional M1 paired with electrical stimuli to the median nerve at the nonparetic wrist. Three suppressive ISIs plus a sham condition were compared. The dependent variable was cortical excitability measured by peak-to-peak amplitude of motor evoked potentials over time.
Results: Two participants with cortical lesions exhibited significant overall suppression of cortical excitability with an ISI of N20-7ms, whereas a participant with basal ganglia lesion showed significant facilitation with the same ISI.
Conclusions: PAS suppressed cortical excitability in cortical stroke but facilitated excitability in basal ganglia stroke. The divergent responses may stem from literature accounts of metaplasticity differences involving individualized intracellular calcium oscillations around thresholds that govern the magnitude and direction of synaptic plasticity to maintain synaptic homeostasis.
Citation
Frost KL, Chen M, Cassidy JM, Snow LAM, Hodges JS, et al. (2017) Paired Associative Stimulation to Suppress Contralesional Corticospinal Excitability in Three People with Stroke Using N-of-1 Crossover Design. J Neurol Transl Neurosci 5(2): 1084.
Keywords
• Stroke
• Transcranial magnetic stimulation
• Paired associative stimulation
• Neuromodulation
ABBREVIATIONS
rTMS: Repetitive Transcranial Magnetic Stimulation; PAS: Paired Associative Stimulation; ISI: Interstimulus Interval; SEP: Sensory Evoked Potential; M1: Primary Motor Area; RMT: Resting Motor Threshold; TMS: Transcranial Magnetic Stimulation; APB: Abductor Pollicis Brevis; N20: Latency
INTRODUCTION
Stroke is a devastating disorder that kills neurons and, depending on the location and severity of the insult, can leave individuals with major movement deficits. But these deficits are not due entirely to the death of neurons; they are also due to the down-regulation of excitability of surviving neurons [1], stemming from deafferentation [2], learned non-use [3], and exaggerated interhemispheric inhibition [4]. As reviewed by Cassidy and Cramer [5], a number of restorative therapies with different purported mechanisms have evolved over the years to restore functional movement, some with good evidence and some not. Importantly, for some people with stroke there may be viable cortical motor neurons in the stroke hemisphere that can be returned to a level of excitability that allows for their voluntary recruitment into functional motor plans. The key is to find the most efficacious methods with which to promote this resurgence as well as the stroke characteristics of those individuals most likely to respond, which amounts to individualized rehabilitation.
Repetitive transcranial magnetic stimulation (rTMS) has been used to modulate cortical excitability following stroke but meta-analyses have shown mixed results with benefits [6,7] and no benefits [8]. Paired associative stimulation (PAS), involving peripheral nerve stimuli paired with cortical stimuli to induce spike-timing-dependent-like shifts in corticospinal excitability [9], may be a more potent alternative to rTMS. For PAS, the directional change in excitability depends on the interstimulus interval (ISI). When a cortical stimulus is applied shortly before the arrival a sensory evoked potential (SEP), suppressive aftereffects occur. When a cortical stimulus is applied shortly after the arrival of an SEP, facilitatory aftereffects occur. PAS has been recognized for its potential to promote motor, sensory and cognitive performance in humans [9]. However, PAS remains under-explored as a potential neuromodulation strategy for hand recovery following stroke, as only two studies [10,11] could be found in the literature and both were directed to promote facilitation, whereas our study was directed to promote suppression. We pursued a strategy of suppressing the contralesional primary motor area (M1) to counter the exaggerated interhemispheric inhibition of the contralesional hemisphere acting on the ipsilesional hemisphere in stroke [4].
Although PAS can have potent effects on neural excitability in responding subjects, it also has wide variability across subjects [12]. Such variability in healthy subjects is likely to be even more pronounced in people with stroke because of gross and subtle differences in their pathology [13]. Dealing with human variability in health science research is a well-recognized problem and N-of-1 trials, defined as single-participant trials using crossover experiments to compare two or more treatments [14], are being used to help promote individualized, precision medicine [15,16]. Accordingly, the purpose of this study was to explore the effects of four different ISIs to suppress excitability of the contralesional M1 in three people with stroke.
MATERIALS AND METHODS
Design
We used an N-of-1 [14], blinded, crossover design with random assignment of four treatment arms and a washout period of at least one week between treatment arms.
Participants
We recruited three people with stroke from our database of participants who had volunteered previously and expressed willingness to participate in future studies. Inclusion criteria were adults with cortical or sub cortical ischemic stroke at least 6 months in duration who were cognitively intact (Mini-Mental State Exam score ≥ 25) with motor impairment in the paretic hand but with at least 10 degrees of active finger extension. The primary exclusion criteria were seizure within the past two years and indwelling medical device. All participants gave consent and the study was approved by the Institutional Review Board.
Testing
Using Ag-AgCl electrodes, the hotspot and resting motor threshold (RMT) for the non-paretic abductor pollicis brevis (APB) were found using single pulses of transcranial magnetic stimulation (TMS) from a 70-mm figure-of-eight coil connected to a Magstim 2002 stimulator (Spring Gardens, Whit land, UK). The contralesional hotspot was the location at which 5 of 10 motor evoked potentials (MEPs) of at least 50µV could be elicited with the lowest stimulator output. The baseline consisted of 30 TMS pulses (0.1Hz) at 130% RMT over the hotspot. The posttest was identical beginning at three minutes after the intervention (time 0) and repeated at 10, 20, 30, 40, 50 and 60 minutes thereafter.
We did not do any testing of changes in behavioral performance because we did not include any rehabilitation training. We excluded rehabilitation training at this stage of exploring PAS in stroke to disentangle the effects of neuromodulation on cortical excitability from possible complications associated with type, intensity, and timing of rehabilitation training. Without accompanying rehabilitation training, there would not be a fair assessment of behavioral effects.
PAS
At each visit, we first determined the participant’s latency (N20) between an electrical stimulus applied to the median nerve at the nonparetic wrist with a Grass electrical stimulator (Natus Neurology Inc., Warwick, Rhode Island, USA) and the detection of the SEP in the contralesional somatosensory cortex with EEG electrodes, as described elsewhere [12]. We then applied contralesional PAS at one of the following ISIs: N20-3ms, N20- 5ms, N20-7ms. Previous work on healthy participants [17] used N20-5ms to induce suppression. We explored N20-3ms and N20- 7ms in addition to N20-5ms because people with stroke may respond differently. The fourth ISI was N20+100ms, considered a sham [18]. Pairs of stimuli (N=225) were then delivered at 0.25 Hz to the hotspot magnetically at 130% of RMT and to the median nerve electrically at 300% of perceptual threshold. Unlike Castel-Lacanal et al., [10,11], we did not direct participants to focus their attention on the paretic hand during the PAS, which is known to increase facilitatory effects of PAS[19], because our PAS intervention was intended to promote suppression of excitability, not facilitation.
Data Analysis
Because peak-to-peak MEP amplitude measurements were asymmetrically distributed at baseline and posttest, we analyzed the common (base 10) logarithm of the MEPs, as done previously [20]. Separately for each participant, we compared between each ISI the grand average change from baseline of the corresponding seven posttest measurements (30 trials x 7 posttests) using a two-way ANOVA. Also, we compared within each ISI the average change from baseline at each posttest time point. We then back-transformed the data to reflect percent change from baseline in the figures. Because this was an initial exploratory study, p-values were not adjusted for multiple comparisons [21].
RESULTS
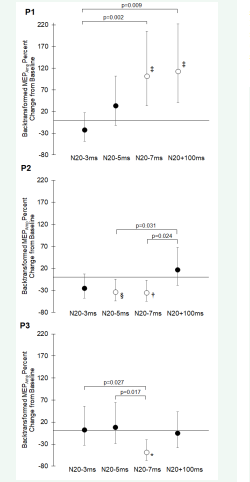
Participant 1 was a 51-year-old female four years post ischemic stroke in the left basal ganglia. No intervention produced a significant suppressive grand average change from baseline excitability (Figure 1).
Figure 1: Grand average (all posttest measurements) of percent change from baseline in motor evoked potential amplitude (MEPAmp) for Participants 1-3 (P1, P2, and P3). Open circles denote significant difference from base line: ‡ (p<0.001), § (p=0.028), † (p=0.019), * (p=0.003). Brackets joining two circles indicate significant differences between conditions. Bars are 95% confidence intervals.
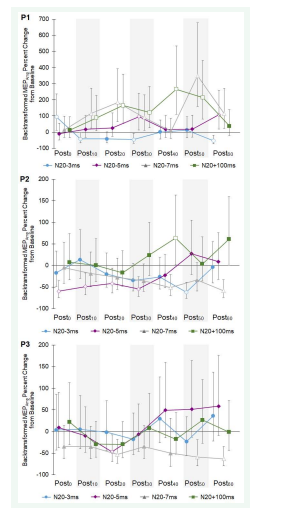
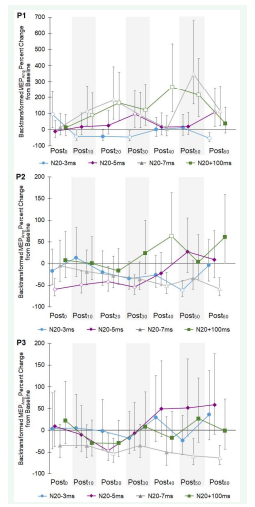
Unexpectedly, the N20+100ms (p< 0.001) and N20-7ms (p< 0.001) ISIs both produced significant facilitatory grand average changes from baseline. Regarding the time course of changes, only N20-3ms showed occasions of the hypothesized significant suppression but these were only sporadic instances with no two consecutive posttests showing significant suppression (Figure 2&3).
Figure 2: Time course of motor evoked potential amplitude (MEPAmp) expressed as percent change from baseline for Participants 1-3 (P1, P2, P3) across seven posttests (Postminutes). Each symbol = average of 30 peak-to-peak MEP amplitudes. Open symbols denote significant difference from baseline (p<0.05). Bars are 95% confidence intervals.
Figure 3: Time course of motor evoked potential amplitude (MEPAmp) expressed as percent change from base line for Participants 1-3 (P1, P2, P3) across seven posttests (Postminutes). Each symbol = average of 30 peak-to-peak MEP amplitudes. Open symbols denote significant difference from baseline (p<0.05). Bars are 95% confidence intervals
Participant 2 was a 58-year-old female 16 years post ischemic stroke in the right frontoparietal cortex. She exhibited suppressive grand average changes from baseline following N20- 5ms and N20-7ms, which were also significantly different from sham (Figure 1). The time courses of these ISI responses showed significant suppression in the early posttests for N20-5ms and in the later posttests for N20-7ms (Figure 2).
Participant 3 was a 64-year-old male 13 years post ischemic stroke in the right temporoparietal cortex. N20-7ms was the only ISI to produce a significant suppressive grand average change from baseline, which was also significantly different from sham (Figure 1). The time course for N20-7ms showed significant suppression primarily near the end of the posttest period (Figure 2). There were no adverse events in any of the participants.
DISCUSSION
This study compared different ISIs in applying PAS to suppress cortical excitability in contralesional M1 of people with stroke. This is relevant because reducing exaggerated interhemispheric inhibition from contralesional M1 acting on ipsilesional M1 with rTMS has been shown to be effective in promoting recovery from stroke [22]; yet PAS, with its rich spike-timing-dependency mechanism for inducing lasting neuroplasticity [9], could possibly be more potent.
The primary finding was that participants 2 and 3, both with cortical stroke, showed significant PAS-induced grand average suppression of contralesional excitability from baseline that was greatest with the N20-7ms ISI. Although we restricted our excitability measurements to only the hemisphere that was stimulated (contralesional), Jayaram and Stinear [23,24] found suppression of contralesional excitability as well as facilitation of ipsilesional excitability when suppressive PAS was applied to the contralesional lower limb motor cortex during walking. Together, these data suggest that suppressive PAS can alter the imbalance of interhemispheric inhibition in stroke [4], but not in all patients as participant 1 in our study, with basal ganglia stroke, paradoxically showed significant facilitation rather than suppression.
It is known that lesion location influences response to intervention [25]. Monte-Silva et al. [26], showed that the amount of dopamine in the cortex influences cortical plasticity and MEP amplitude. Thus, it is possible that the absence of a grand average suppressive effect in the participant with basal ganglia stroke may be related to reduced dopamine.
More likely, given that variability of excitability measurements plagues most neuromodulation studies, the deviation between the participant with basal ganglia stroke and the two with cortical stroke may relate to metaplasticity. High variability in neuromodulation responses within and between people, including both magnitude and direction of response, is well known and is likely multifactorial in origin [27]. Hamada et al. [28], discuss several studies in which healthy subjects and neurological patients respond to theta-burst stimulation with conflicting directional responses. Through TMS experiments involving different figure-of-eight coil directions, they concluded that conflicting responses may not reflect differing capacities for cortical plasticity but rather reflect differences in the time-varying recruitment of interneuron networks by each TMS pulse. Physiologically, Fung et al. [29,30], suggest that such divergence in responsiveness may stem from intracellular calcium oscillations around thresholds associated with ongoing metaplasticity to maintain synaptic homeostasis. Such individualized time-varying differences in metaplasticity may explain the noisy responsiveness observed by each participant in Figure 2 as well as the deviant directions of the significant grand average changes from baseline of participant 1 in Figure 1.
The overall clinical implications of the significant grand average responses for participants 2 and 3 are that suppressive PAS may hold value in modulating cortical excitability and could thus be considered as an adjunct to conventional rehabilitation to achieve higher functional outcomes. But the optimal ISI still needs larger exploration, which could indicate that, because of inherent differences in metaplasticty, optimal ISIs need to be individualized. Also, the time course of the PAS after-effects requires further exploration. Figure 2 shows that the significant suppression for one ISI occurred early in the posttest period whereas for another it appeared late, which would influence the optimal time for implementing the rehabilitation training following the PAS.
Limitations
Because of the multiple comparisons of the average change from baseline at each time point within each ISI without Bonferroni correction, the possibility for type I statistical error is elevated and so these results must be considered with caution. With so few participants, generalizability of our findings is not possible and so we cannot conclude that N20-7ms is the optimal ISI to be used throughout stroke. As explained above, no assessments of behavioral change were made at this stage but need to be made once reasoned PAS protocols for influencing cortical excitability in stroke have been formed.
CONCLUSION
We conclude that PAS with an ISI of N20-7ms produced the intended grand average suppression of excitability in the two participants with cortical lesions but not in the participant with a basal ganglia lesion. Because of inherent oscillations in intracellular calcium levels that govern the magnitude and direction of synaptic plasticity [29,30], the most effective approach for applying PAS in stroke requires continued exploration in an effort to stabilize response variability and more confidently prod synaptic plasticity in the intended direction, ultimately leading to higher functional recovery.
ACKNOWLEDGEMENTS
This project received support from the University of Minnesota’s Institute for Engineering in Medicine Group Grant Program, the University of Minnesota’s MnDRIVE (Minnesota’s Discovery, Research and Innovation Economy) initiative, the National Center for Advancing Translational Sciences of the National Institutes of Health (Award Number UL1TR000114), and from the National Center for Research Resources of the National Institutes of Health to the University of Minnesota Clinical and Translational Science Institute (Award Number 1UL1RR033183). We acknowledge the valuable contributions from Enny Akinsooto, DPT: Erin Babineau, DPT: Grant Blasczyk, DPT: Kaitlin Brendel, DPT: Brittany Burton, DPT: Danielle Busch, DPT: and Taylor Thompson, DPT.