Relationships between Praxis Skills and Neural Organization of Grammar
- 1. Department of Communication Sciences and Disorders, Idaho State University, USA
Abstract
Currently, significant gaps remain in our knowledge regarding the connection between language and praxis skills. However, literature has shown that similar or overlapping neural structures appear to be involved in language and grammar function, and praxis skills; incidentally, grammatical deficits co-occur at a high rate with ideomotor apraxia when inferior frontal language regions are damaged. While the planning of phonetic speech gestures and grammar are logically separate skills and can be dissociated, this theoretical review discusses important but often overlooked commonalities between these abilities; namely, the fact that they both involve implicitly learned sequencing of abstract units. This review further highlights research in support of the position that frontal association areas coordinate the domain specific abilities of grammar, syntax, and gestures whose representations lie in separate brain regions.
Keywords
• Aphasia
• Apraxia of speech
• Ideomotor apraxia
• Broca’s area
• Language
• Syntax
Citation
Nicholas AJ, Seikel A, Hudock D (2015) Relationships between Praxis Skills and Neural Organization of Grammar. J Neurol Transl Neurosci 3(2): 1058
Introduction
Research on praxis abilities has demonstrated that the neurophysiological systems involved in motor learning and sequencing encompasses multiple brain regions. These include the prefrontal and motor cortices, striatum, cerebellum, left angular gyrus, and supra-marginal gyrus [1-3]. While few studies have closely examined the relationship between praxis and language capabilities, and those that have demonstrate a correlation between praxis skills and language capabilities subsequent to stroke [4].
In fact, the high co-occurrence of language deficits such as grammatical deficits along with ideomotor apraxia, as suggested by behavioral and neuroimaging evidence, has led to the speculation of the existence of overlapping brain regions governing speech, language, and other praxis skills [5]. This report will examine the implications of these observations: The central thesis of this review is that shared circuits in frontal association regions simultaneously operate on separate structures associated with language and praxis respectively. Hence, we propose that a hierarchically organized structure controls sequencing or “syntactical” operations across multiple domains of cognition including motor programs and grammar. The term “syntax” may be defined as a set of cognitive operations responsible for sequencing discrete units or representations— phonetic sequences, words, etc.—into grammatically acceptable strings using native language rules [6,7]. Most relevant to this article, these operations consist of grammar and language production sequences, but also may include written words and even syntax in music as is described later.
The following section examines the mounting evidence showing that overlapping brain functions underlie speech production skills, as well as syntactic processing in grammar. One important consequence of these observations is that Broca’s area not only plays a crucial role in coordinating the musculature involved in speech production, but also that this region and associated structures in the motor cortex contain grammatical and syntactic processing circuits operating on inputs across modalities. As we shall see, evidence has increasingly indicated that domain specific sequencing functions are sometimes operated upon by a unified set of cognitive resources in frontal brain regions. Both behavioral as well as recent evidence from neuroimaging studies will described in relation to the commonalities of praxis skills and language.
Neural Structures for Language and Praxis
In terms of receptive language, it is well-known that people with experience in a specific language are often capable of detecting deviations from grammatical patterns found in their native language and errors in music respectively [8,9]. An important implication is that grammatical (i.e., sequencing) errors that violate the rules of a given language tend to stand out to the observer (e.g., “John put book the table on,” rather than “John put the book on the table”).
Importantly, the ability to implicitly categorize a string of items as “correct” versus “incorrect” goes beyond the confines of language and grammar; this happens to be a capability that even extends to sequences of individual notes and chord progressions in musical compositions [7]. For example, neuropsychological tests have shown that musical and language abilities are represented in separate neural circuits where musical and speech production abilities can be dissociated in clinical populations [7]. Nonetheless, Patel reported evidence from neuroimaging studies indicating that shared resources in frontal brain regions operate on these separate circuits. Using information from the neuroimaging literature and individual case studies of patients with Broca’s aphasia, proposed that domain specific processes, in this case language and musical representations, are controlled hierarchically by syntactical functions in frontal brain areas in real time [7]. This leads to the following prediction: music and language skills— perception, production, and repetition—can be dissociated. However, patients with Broca’s aphasia should predictably experience deficits beyond language production skills that extend into musical syntax and grammar more generally. Similar to music and language representations, we argue that grammatical and motor speech (i.e., phonetic) representations lie in separate neural circuits; however, shared resources in frontal areas operate on these representations. We argue that support for this position comes from the fact that damage to inferior frontal regions, such as Broca’s area, commonly leads to grammatical and phonetic speech deficits [4,5,10]. This is crucial, as it appears that damage to these overlapping frontal brain circuits will lead to a triad of language and praxis dysfunctions including motor sequencing, gesture repetition, and deficits in grammar across a variety of domains.
Broca’s Area and Sequencing Abilities
Research has shown that Broca’s region and other language processing areas such as Wernicke’s operate upon implicitly learned syntactic and grammatical representations; such functions exist in addition to implementing motor speech plans and comprehending spoken words respectively. One imaging study in particular reported evidence for a significant role of implicit grammar processing in left hemispheric brain regions devoted to speech perception and production [11]. The authors reported an experiment involving of visual word recognition using magneto encephalography (MEG). They observed, as initially expected, significant changes in occipital lobe activity when observers viewed real words and nonsense words compared to baseline. On the other hand, Broca’s and Wernicke’s areas responded similarly to word stimuli, and furthermore, showed signs of implicit word processing strategies such as visual search. Additionally, in another study investigating brain regions sub serving grammar learning, Petersson and colleagues administered a grammatical classification task (“acceptable” vs. “unacceptable”) to participants subsequent to viewing examples of an artificial grammar [12]. Artificial grammars contain a set of rules that generates a string of alphanumeric units. In a typical experiment, participants are unaware of the exact rules; however, they can often learn the rules implicitly after a study phase where they see several exemplars of correct stings. Importantly, the fMRI imaging results in this study indicated that Broca’s area was consistently activated during grammatical checks, suggesting that this region operates on abstract (not just auditory) units and is involved in grammatical functions at some level.
Together, these findings strongly suggest an overlap in circuitry involved in learning strings of novel sequence in addition to language perception and production. Damage to these regions (i.e., Broca’s area, or Brodmann’s area 44 and potentially area 45) particularly in the left hemisphere for most individuals, will generally cause deficits in both grammar and speech production [4,5]. As we shall demonstrate in the following section, these points have implications for theories of cognitive speech and language disorders: in particular, for aphasia and apraxia of speech. This section discusses how damage to Broca’s area disrupts implicit functions governing speech production and grammar. Crucially, we argue that the high co-occurrence between apraxia of speech and non-fluent aphasia can be explained by the fact that grammatical skills and speech motor planning functions are carried out by overlapping brain areas.
Implications for Speech Perception and Production
First, Figure 1
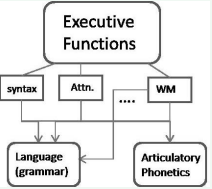
Figure 1 Framework depicting the implementation of a speech production command. While grammatical and articulatory phonetic representations exist in separate circuits, they both are controlled by frontal executive function circuitry encompassing abilities such as working memory (WM), attention (Attn.) and syntax/grammar.
diagrams an illustration of the domain specific circuits of language and praxis. According to our position, these circuits are operated upon hierarchically by frontal brain regions, namely Broca’s and other motor association areas in typical cases. This is why damage to left inferior areas generally causes a cascade of deficits affecting both praxis and grammar in the majority of listeners. While hemispheric dominance for language and praxis are correlated [13], Kobayashi and Ugawa reviewed evidence on lateralization and noted that stored programs for praxis movements (i.e., the so called “praxicon”) are typically confined to the left hemisphere independent of handedness [5]. The fact that language and grammar on one hand, and the praxicon, on the other, can exist independently may explain some of the reported dissociations between language and praxis skills reported in the literature.
Ideomotor apraxia and non-fluent aphasia are therefore logically distinct, and apraxia has been shown to occasionally arise independently of aphasia (incidentally, some forms of aphasia may also exist independently of oral apraxia as well [4]. However, it is important to note that aphasia and apraxia share more in common than previously realized; both are characterized by acquired dysfunction of implicitly learned sequences of items.
Information suggesting an intimate connection between language and praxis is not entirely new. In fact, research going back to the 1960’s reveals a robust positive correlation between oral praxis and phonemic-articulations skills in patients with left hemispheric strokes [10]. The association is especially strong between diagnoses of apraxia of speech and Broca’s aphasia as we would expect, although again, there were reportedly some examples of patients with ideomotor oral apraxia without aphasia. Historically, apraxia of speech and non-fluent aphasia has been shown to display a high rate of co-occurrence and remarkably similar characteristics, namely articulatory (praxis) difficulties along with grammatical and syntactic deficits (language) [14,15]. Articulation difficulties observed in ideomotor apraxia are characterized by limited verbal output, reduction in the use of function words, and articulatory phonetic errors. Grammatical difficulties manifest in a more domain general manner; it often involves deficits in one’s ability to identify or produce correct strings of units. Crucially, articulatory dysfunction may be associated with grammatical deficiency because both involve the inability of frontal brain areas to correctly retrieved implicitly learned sequences. The explanation for these findings appears multifaceted. First, the co-occurring grammatical and speech production deficits have been shown to be caused by overlapping circuitry involved in conveying commands to the glossopharyngeal musculature responsible for instantiating implicitly learned speech production sequences [4,10,16-20].
Second, frontal brain areas also subsume implicitly learned sequencing functions associated with grammar, including those associated with visual language processing and music as previously described. Hence, one way to reframe this idea is to propose that the shared circuitry is a metalinguistic grammar processor that operates on units (e.g., words, phonemes, gestures) irrespective of domain.
Overlapping neural circuitry
When articulatory deficits co-occur with phonemic, syntactic or grammatical problems, damage to overlapping brain circuits in inferior frontal brain areas is generally observed [21]. In most instances, the etiology specifically appears to be damage extending into inferior left frontal regions anterior to the motor strip. One may therefore view brain areas in the proximity of Brodmann’s area 44 and other speech production areas as major brain regions associated with programming the form of speech production. This ranges from the elemental level of articulatory gesture all the way to modulation of grammatical and syntactic structures [22]. Lesions to the Broca’s area, and insular cortex within the language dominant hemisphere result in varying degrees of motor planning and sequencing deficits. Broca’s area could be viewed as responsible for generating the basic form of the phoneme, with the superordinate tissue of the pars triangularis supporting morphemic and syntactic form [23].
A view related to that discussed above in the preceding paragraph, known as the dual-rout hypothesis, might also explain characteristics of the sequencing deficits observed in patients with ideomotor apraxia and certain types of aphasia (see [5] for further discussion). A key aspect of this hypothesis is that two distinct pathways—a ventral and a dorsal—intersect in Broca’s area. The ventral pathway is involved in input or sensory identification (i.e., the “what” pathway), and projects to Broca’s area via the superior temporal gyrus and also to the operculum. The dorsal pathway is involved in spatial relations and hence is often referred to as the “where” pathway; this projects to Broca’s and pre-motor areas through a route from the superior temporal gyrus. A second part of this hypothesis is that language, and praxis more generally, encompasses a combination of symbolic and motor-kinematic skills. That is, both language and praxis involve the identification of correct sequences (“what”) and also the instantiation language/praxis processes using the correct motor sequences (“where”) [24], for a historical review of the visual system). In certain cases these processes are dissociable: Damage to the ventral pathway can lead to sensory aphasia and inability to recognize the correct sequence of movements, especially when tool use is involved. Conversely, damage only to the dorsal pathway usually leaves recognition intact, but the inability to produce the correct sequences—speech related or otherwise—are compromised. However, damage directed to Broca’s area compromises the correct functioning of both pathways to some extent.
In the following subsection, we will briefly examine the sequencing or “production” deficits that arise in production deficits characteristic apraxia and non-fluent aphasia. The subsequent subsection will focus on the receptive and productive components of grammar.
Speech Production
Neurological insult to Broca’s area and the underlying white matter often contributes to a failure to formulate or implement output. Damage associated with apraxia of speech or non-fluent aphasia does not involve impairment of muscle coordination or the inability for the glossopharyngeal apparatus to carry out speech motor commands per se as is the case with dysarthria. Rather, the deficit suggests a disability in translating abstract programs into actual speech-motor sequences or gestures [25] for a discussion on motor planning vs. programming.
For illustration purposes, Figure 2
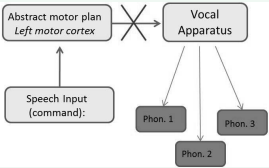
Figure 2 Framework depicting the implementation of a speech production command. For example, a speaker may intend to say the word “bed” (phonetic gesture 1 = “b”, 2 = “e”, and 3 = “d”). In apraxia of speech, the formulation of the command occurs through speech production circuits. However, the destruction or developmental malformation of neural connections from the motor cortex to the speech production apparatus yields disrupted articulation or the absence of articulation in more severe cases
shows a possible type of disruption of the translation of abstract motor commands into executable programs. This diagram specifically applies to speech, but similar principles should apply to actions involving other gestures and correct sequences of tool use. Here, the disruption of the flow of information originates subsequent to the formation of the output instructions during which the instructions fail to be translated into a motor command. As expected, studies employing measurements of articulatory precision have identified abnormal motor control in people with apraxia compared to normal speakers. However, several studies have also observed less impairment compared to patients diagnosed only with dysarthria [26,27].
The suggested framework in Figure 2 assumes a serial process through which phonetic representations are selected from a repertoire of sequences in the form of stored lexical representations [28,29]. A motor plan is then formulated in the premotor cortex, and finally, an output is translated and generated in real time by the glossopharyngeal apparatus. Speech production at the level of the sentence can be accomplished by sequencing phonetic output in grammatically correct units, although a grammar generating stage is not directly built into this framework. Such an augmentation may be made through the addition of a separate stage in which the phonetic sequences are interfaced with a grammar system that checks such sequences for their acceptability. One aspect of this framework is that it does not predict feedback between stages or processes other serial accounts of speech production, refer to [29-32]. For stage 1, the assumption is that lexical and phonemic representations are stored in neural networks and encoded in long-term memory, and that damage to a variety of left hemispheric language circuits could produce word finding deficits (i.e., anomic aphasia).
To summarize, production deficits can be due to the inability to select the correct lexical representation, formulate the output (in which case the ‘X’ in Figure 2 would be placed over the box labeled ‘Abstract Motor Plan’), or translate the instructions formulated the motor cortex and Broca’s area to a concrete set of output instructions (the actual location of the ‘X’ in Figure 2 [26]. In terms of neurological structures, evidence suggests the precentral gyrus of the left insula plays a primary role in the instantiation of complex articulatory gestures before the final execution of speech motor commands [33]. This framework appears to find considerable support in a growing body of literature assuming the integration of motor programing, representational, and phonological systems in a unified framework [32,34](Scott et al., 2009; Ziegler et al., 2012).
In short, our suggested approach draws attention to the proximal link between neural circuitry responsible for praxis skills and language production; it predicts that damage near Broca’s area will often result in apraxia [35,36]. Once again, this latter result is evidenced by the high co-occurrence of non-fluent aphasia, apraxia of speech, and other forms of apraxia [4,37-39].
Grammar
Praxis and grammar skills share a relationship. For example, it has been shown that patients with praxis deficits often display implicitly learned sequencing deficits that impinge on language and grammar skills [4,14]. Deficits in grammar are generally referred to as a grammatism and defined as the general loss of function words necessary for sequencing wordsin a grammatically acceptable language sentence; this deficit may involve the inability to identify a correct sequence when presented with one, produce a correct sequence of units, or both [14,22,40]. Overall, grammatical dysfunction points to either the loss of or difficulty accessing syntactic-grammatical representations. Such deficits are pervasive among speakers with apraxia [4-44].
In a seminal study, Berndt and Caramazza reviewed both the grammatical and syntactic deficits in patients diagnosed with ideomotor (oral) apraxia and non-fluent aphasia [14]. Deficits were commonly characterized by the reduced or nearly absent production of function words and nouns necessary for proper formation of grammatical or syntactical structures. These results suggested that Broca’s region and underlying white matter connections via the arcuate fasciculus play a role in sequencing and implicitly learned functions beyond phoneme production— even extending into the domain of music as previously discussed [7]. Hence, there is evidence for a shared neural linguistic sub-system, and also that the substrates responsible for grammatical expression are often recruited for rule-based decoding. Current evidence indicates the existence of circuits responsible for syntactic representations and processing in inferior frontal areas and the left temporal lobe respectively [22,45]. In another landmark study, repetitive Trans-Cranial Magnetic Stimulation (rTMS) studies have administered inhibitory pulses over Broca’s area in healthy participants and reported evidence for deficits in both syntactic perception and production as we would expect from the dual process model [45].
Additionally, studies investigating sentence repetition have long shown evidence for similar syntactic level errors in patients with stoke damage to inferior frontal regions [46]. More recent findings have been uncovered in the adult literature on apraxia: Dovern and colleagues for example, investigated sequential motor learning in left hemispheric stroke patients, both with and without verbal apraxia, in an incidental serial response task [17]. Participants spent four blocks learning six-element sequences of motor commands before being introduced to a novel sequence in the fifth block. Stroke patients with apraxia exhibited slower reaction times compared to control participants, and perhaps even more significantly, poorer purposeful retrieval of implicitly learned sequences. These data provide important clues into the ability of patients with apraxia to encode and retrieve implicitly learned sequences, including phonological and grammatical forms.
Interestingly, these results indicate that the retrieval of incidentally learned sequences may be adversely affected to a greater degree than the stored representations themselves, the latter of which includes the ability to learn motor commands implicitly. We therefore propose that more severe damage will manifest in an inability to retrieve and implement commands necessary for sequential word production, while less severe damage should most often lead to milder sequencing errors, that is, milder phonetic and prosody related errors.
Together, these studies motivate the prediction that damage to “language processing zones” will yield disrupted syntactic processing abilities and the abnormal sequencing of linguistic units. (Conversely, patients with fluent aphasia have more difficulty accessing certain nouns, and may overuse function words or circumlocution when attempting to name an object, thus showing signs of para-grammatism.). These observations of deficits of syntax and grammar have not surprisingly contributed to the debate about whether damage to Broca’s area causes the loss of syntactic representations, or alternatively, whether patients with aphasia simply have difficulty accessing syntactic representations that could in principle be un-blocked [14,28]. We do not aim to settle the debate about whether non-fluent aphasia contributes to the loss of syntactic representations themselves, or instead, whether damage causes loss of access to the representations. Distinguishing between these two possibilities is empirically difficult. We only propose that both cases are possible, perhaps with more severe cases leading to loss of representations and milder cases contributing to a loss of access. The observation that patients with apraxia of speech often lose the ability to combine phonemes, words, and syllables linguistic components into meaningful phrases, in addition to deficiencies in comprehending complex grammatical constructions beyond isolated nouns, appears to suggest that the syntactic representations themselves could be impaired in certain cases. Interestingly, grammar and other executive and left hemispheric functions are closely related; verbal working memory skills for instance, have been shown to be positively correlated with syntactic processing skills. Likewise, deficiencies in syntactic constructions in written language also support the hypothesis that the loss of syntactic representations occurs in some individuals with non-fluent aphasia and apraxia [41,47].
SUMMARY AND CONCLUSIONS
The primary aim of this review paper was to explore the relationship motor production deficits and grammatical/ syntactic deficits that co-occur at a high rate in patients with apraxia, particularly apraxia of speech, and non-fluent aphasia. We pointed out that there appears to be a paucity of research directly comparing and contrasting the qualities of each disorder, although noteworthy exceptions—both recent on going back several decades to the 1960s—were noted. Importantly, the available research has consistently shown a strong association between praxis and language skills in stroke patients. Our report further aimed to explain this relationship in light of relatively rare dissociations between praxis and language deficits (i.e., between apraxia and aphasia). The explanation provided was akin to a framework provided by Patel to explain how on one hand, musical and language syntax may be dissociated, yet be operated upon by shared frontal lobe resources [7]. We extended this conceptualization to argue that frontal circuitry—dominated by Broca’s area—functions as a metalinguistic grammar control unit that operates on domain specific praxis and grammatical.
Accordingly, damage to Broca’s area should cause cascading deficits in most cases affecting grammar and speech production. Differential damage in certain cases, nonetheless, may cause aphasias without oral apraxia, or apraxia without aphasia depending on the hemispheric specialization and distribution of this specialized circuitry. Our position generates testable predictions useful for future research: while pure forms of apraxia exist apart from non-fluent aphasia, and certain forms of aphasia (e.g., conduction, transcortical, and Wernicke’s) can exist without apraxia, non-fluent aphasia should always result in a combination of motor sequencing and grammatical deficits.
Finally, for future directions and applications, we suggest that measures of implicit grammar learning should be incorporated into standardized tests of apraxia of speech. Testing on patients with suspected apraxia or aphasia should therefore gauge grammar skills and implicit learning capacity using standardized verbal or language material, and include measures of articulatory-phonetic production of phoneme, word, or syllable sequences [33]. In particular, we suggest incorporating and developing standardized speeded-processing tests of implicit learning or grammar; this is because processing speed has proven to be a sensitive indicator of subtle differences in information processing and cognitive abilities in cued memory tasks [48].
ACKNOWLEDGMENTS
The project described was supported by the INBRE Program, NIH Grant Nos. P20 RR016454 (National Center for Research Resources) and P20 GM103408 (National Institute of General Medical Sciences).
REFERENCES
6. Jackendoff R. Foundations of Language. Oxford University Press: New York, NY. 2002.
7. Patel AD. Language, music, syntax and the brain. Nat Neurosci. 2003; 6: 674-681.
8. Pinker S. The Language Instinct. Harper Perennial Modern Classics: New York, New York. 1994.
9. Sloboda J. The Musical Mind: The Cognitive Psychology of Music. Oxford University Press. 1985.
10. De Renzi E, Pieczuro A, Vignolo LA. Oral apraxia and aphasia. Cortex. 1966; 2: 50-73.
15. Helm-Estabrooks N, Albert ML Manual of Aphasia and Aphasia Therapy. Austin, TX: Pro-Ed. 2004.
16. Darley FL, Aronson AE, Brown JR. Motor speech disorders. Philadelphia: Saunders. 1975.
19. Heilman KM. Apraxia. Continuum (Minneap Minn). 2010; 16: 86-98.






































































































































