Scoring System Approach of Reverse Nutech Functional Score to Assess Patients with Multiple Sclerosis
- 1. Nutech Mediworld, Green Park Extension, India
Abstract
Background: Multiple sclerosis (MS) is a common neurological disease characterized by recurrent relapses within the central nervous system (CNS). Several pathophysiological mechanisms such as axonal/neuronal damage, demyelination, inflammation, gliosis, remyelination and repair, oxidative stress and excitotoxicity, alteration of the immune system and disruption of blood-brain barrier are involved in the progression of disease. Various diagnostic tests and scoring scales are used for the diagnosis of MS in patients, but the findings of these tests and scales could not prove to be completely valid. This study aimed at developing a scoring system to assess the patients suffering from MS.
Methods: After assessing the list of recorded symptoms, a single list that includes all the possible symptoms associated with MS, a Reverse Nutech Functional Score (RNFS) for MS is built. To facilitate the conduct of probability based studies, we have tried to convert the categorized grades to numeric values in the range of (0,1).
Results: We established a scoring system, Nutech Functional Score (NFS), which is a 36 point scoring system that evaluates the patients with MS both before and after human embryonic stem cell (hESC) therapy. Each symptom is graded as (1,2,3,4,5) that runs in GOOD → BAD direction.
Conclusion: RNFS is a beneficial scoring system that can be used worldwide to assess the patients with MS. No other system provides a valid numeric value regarding the condition of patient at a given time.
Keywords
• Multiple sclerosis
• Reverse Nutech Functional score (RNFS)
• Scoring system
• Expanded Disability Status Scale (EDSS)
• Diagnosis
Citation
Shroff G (2016) Scoring System Approach of Reverse Nutech Functional Score to Assess Patients with Multiple Sclerosis. J Neurol Transl Neurosci 4(2): 1066.
ABBREVIATIONS
MS: Multiple Sclerosis; CNS: Central Nervous System; EDSS: Expanded Disability Status Scale; FS: Functional System; FSS: Functional System Score; AI: Ambulation Index; SNRS: Scripps Neurological Rating Scale; ISS: Illness Severity Scale; GNDS: Guy’s Neurological Disability Scale; MSIS: Multiple Sclerosis Impairment Scale; CAMBS: Cambridge Multiple Sclerosis Basic Scores; MSQOL: Multiple Sclerosis Quality of Life; MSQOLI: Multiple Sclerosis Quality of Life Inventory; RNFS: Reverse Nutech Functional Score; IEC: Institutional Ethics Committee; NAA: Not Afflicted in Ailment; NE: Not Existing; MRI: Magnetic Resonance Imaging; CT: Computed Tomography; CFA: Cerebrospinal Fluid Analysis; ADL: Activities of Daily Living
INTRODUCTION
Multiple sclerosis (MS) is a chronic inflammatory disorder of the central nervous system (CNS) that causes non-traumatic disability among young and middle-aged adults [1]. It affects around 2.5 million people worldwide and is the third most common neurologic disorder cited as the cause of disability [2,3].
In recent decades, a number of scales have been developed to examine the clinical severity and the functional deficits of patients with MS. These scales are increasingly used as an endpoint in clinical trials to assess the effectiveness of therapeutic interventions. Most frequently used scale for the evaluation of disability in MS is the Expanded Disability Status Scale (EDSS). Basically, EDSS quantifies disability in eight Functional Systems (FS) and allows neurologists to assign a FS Score (FSS) in each of this system. EDSS is a clinician-administered assessment scale evaluating the FS of the CNS. EDSS is used to describe the disease progression in patients with MS. It consists of ordinal rating system ranging from 0 (normal neurological status) to 10 (death due to MS) in 0.5 increments interval (when reaching EDSS 1). The lower scale values of the EDSS measure impairments are based on the neurological examination, while the upper range of the scale (>EDSS 6) measures disability of patients with MS. The determination of EDSS 4 – 6 is heavily dependent on aspects of walking ability [2].
A number of other scales are also available to assess MS: the Ambulation Index (AI) [4], the Scripps Neurological Rating Scale (SNRS) [5], the Illness Severity Scale (ISS) [6], the Guy’s Neurological Disability Scale (GNDS) [7], the Multiple Sclerosis Impact Scale (MSIS-29) [8] and the Multiple Sclerosis Impairment Scale (MSIS) [9]. Furthermore, the Cambridge Multiple Sclerosis Basic Scores (CAMBS) [10] are clinical assessment scores that could be used to assess MS [11]. Specific scales for measuring health-related quality of life in MS patients are the Multiple Sclerosis Quality of Life-54 (MSQOL-54) [12], Functional Assessment of Multiple Sclerosis (FAMS) [13] and the Multiple Sclerosis Quality of Life Inventory (MSQLI) scales [14]. However, only a few of these scales meet the requirements of methodological standards like validity, reliability, responsiveness for use in clinical practice.
Reverse Nutech Functional Score (RNFS) assess the condition of patients with MS based on clinical symptoms. The primary goal for creating the RNFS was to improve the standard measure of MS disability and to develop a metrics of overall clinical status of patients with MS. This scoring system allows monitoring of patients while adopting therapeutic modalities. The current paper will discuss about the development of RNFS for MS and will compare it with EDSS.
MATERIALS AND METHODS
The study was conducted in accordance to the approval provided by the independent institutional ethics committee (IEC) of Nutech Mediworld. The study included patients with MS who either visited directly or were referred to the institute. The patients were either previously diagnosed or were diagnosed at our institute with the medical procedure routinely used for MS. The diagnostic history recorded all the symptoms that were found to be present while evaluating the patients. The institute studied the lists of recorded symptoms and prepared one single list which could be used to evaluate a patient with MS. This list of symptoms was revised from time to time in order to maintain accuracy. The RNFS scoring system evaluates a symptom based on five ordinal grades that runs in GOOD→ BAD direction. To arrive at case-wise average values after conducting probability based studies, the grades were converted into numeric values. All the patients who were previously assessed with EDSS were than assessed with RNFS.
RESULTS
A 36-point scoring system that includes all the possible symptoms that were recorded for MS. (Table 1)
|
Table 1: Reverse Nutech Functional Score for Multiple Sclerosis. |
||
|
Parameter |
Description |
Score |
|
Muscle weakness (Area affected: extremity, back, chest) |
Not afflicted in MS |
NAA |
|
Not existing |
NE |
|
|
More than 3 areas involved |
5 |
|
|
3 areas involved |
4 |
|
|
2 areas involved |
3 |
|
|
1 area involved |
2 |
|
|
Normal |
1 |
|
|
Walking |
|
|
|
Distance |
Not afflicted in MS |
NAA |
|
Not existing |
NE |
|
|
Less than five meters/ cannot walk alone |
5 |
|
|
Can walk up to 25 meters only |
4 |
|
|
Can walk from 50 meters to 100 meters only |
3 |
|
|
Can walk > 100 meters up to < 500 meters only |
2 |
|
|
Can walk normal distances |
1 |
|
|
Walking Aid |
Not afflicted in MS |
NAA |
|
Not existing |
NE |
|
|
Walker with elbow support |
5 |
|
|
Walker |
4 |
|
|
Elbow crutches |
3 |
|
|
Cane |
2 |
|
|
No aid required |
1 |
|
|
Balance |
|
|
|
Eyes closed in straight line |
Not afflicted in MS |
NAA |
|
Not existing |
NE |
|
|
Cannot stand still with eyes closed |
5 |
|
|
Can stand still but cannot walk with eyes closed |
4 |
|
|
Can walk up to 10 steps with eyes closed |
3 |
|
|
Can walk more than 10 steps but not independently |
2 |
|
|
Normal balance restored |
1 |
|
|
Eyes open in straight line |
Not afflicted in MS |
NAA |
|
Not existing |
NE |
|
|
Cannot stand still with eyes open |
5 |
|
|
Can stand still but cannot walk with eyes open |
4 |
|
|
Can walk up to 10 steps with eyes open |
3 |
|
|
Can walk more than 10 steps but not independently |
2 |
|
|
Normal balance restored |
1 |
|
|
Sitting |
Not afflicted in MS |
NAA |
|
Not existing |
NE |
|
|
No sitting balance at all |
5 |
|
|
Requires maximum external support |
4 |
|
|
Requires minimum external support |
3 |
|
|
Sits with no external support |
2 |
|
|
Sitting balance normal |
1 |
|
|
Fatigue |
Not afflicted in MS |
NAA |
|
Not existing |
NE |
|
|
Permanent exhausting fatigue |
5 |
|
|
Fatigue after daily hygiene activities |
4 |
|
|
Fatigue after all normal daily activities |
3 |
|
|
Fatigue after only gentle workout |
2 |
|
|
No fatigue |
1 |
|
|
Stiffness (Areas affected: upper/lower extremities, back, chest) |
Not afflicted in MS |
NAA |
|
Not existing |
NE |
|
|
More than 3 areas involved |
5 |
|
|
3 areas involved |
4 |
|
|
2 areas involved |
3 |
|
|
1 area involved |
2 |
|
|
Stiffness absent |
1 |
|
|
Tremors (Areas affected: lower/upper extremities, back, trunk, head including face) |
Not afflicted in MS |
NAA |
|
Not existing |
NE |
|
|
More than 3 limbs involved |
5 |
|
|
3 limbs involved |
4 |
|
|
2 limbs involved |
3 |
|
|
1 limb involved |
2 |
|
|
Tremors disappeared |
1 |
|
|
Paraesthesia (Areas affected: lower/upper extremities, back, trunk, head including face) |
Not afflicted in MS |
NAA |
|
Not existing |
NE |
|
|
More than 3 limbs involved |
5 |
|
|
3 limbs involved |
4 |
|
|
2 limbs involved |
3 |
|
|
1 limb involved |
2 |
|
|
Paraesthesia disappeared |
1 |
|
|
Pain: Intensity and Type (Stabbing, burning, prickling, tearing, pressure) |
Not afflicted in MS |
NAA |
|
Not existing |
NE |
|
|
More than 3 areas involved |
5 |
|
|
3 areas involved |
4 |
|
|
2 areas involved |
3 |
|
|
1 area involved |
2 |
|
|
Pain absent |
1 |
|
|
Hand functions - Gross |
Not afflicted in MS |
NAA |
|
Not existing |
NE |
|
|
Unable to do |
5 |
|
|
Need maximum assistance |
4 |
|
|
Need moderate assistance |
3 |
|
|
Need minimum assistance |
2 |
|
|
Total independence |
1 |
|
|
Hand Functions - Fine |
Not afflicted in MS |
NAA |
|
Not existing |
NE |
|
|
Unable to do |
5 |
|
|
Need maximum assistance |
4 |
|
|
Need moderate assistance |
3 |
|
|
Need minimum assistance |
2 |
|
|
Total independence |
1 |
|
|
Standing Balance |
Not afflicted in MS |
NAA |
|
Not existing |
NE |
|
|
Cannot stand |
5 |
|
|
Stand with Caliper + Maximum Therapist support |
4 |
|
|
Stand with Caliper + Minimum Therapist support |
3 |
|
|
Stand independently with caliper (with no external support) |
2 |
|
|
Stand normally |
1 |
|
|
Tingling (Areas affected: extremity, back, abdomen, chest, face and head) |
Not afflicted in MS |
NAA |
|
Not existing |
NE |
|
|
More than 3 areas involved |
5 |
|
|
3 areas involved |
4 |
|
|
2 areas involved |
3 |
|
|
1 area involved |
2 |
|
|
No Tingling |
1 |
|
|
Paralysis of upper/lower Extremities |
Not afflicted in MS |
NAA |
|
Not existing |
NE |
|
|
More than 3 limbs involved |
5 |
|
|
3 limbs involved |
4 |
|
|
2 limbs involved |
3 |
|
|
1 limb involved |
2 |
|
|
No Paralysis |
1 |
|
|
Short term Memory |
Not afflicted in MS |
NAA |
|
Not existing |
NE |
|
|
No Short-term memory at all |
5 |
|
|
Severe short-term memory loss |
4 |
|
|
Moderate short-term memory loss |
3 |
|
|
Mild short-term memory loss |
2 |
|
|
Memory becomes normal |
1 |
|
|
Attention |
Not afflicted in MS |
NAA |
|
Not existing |
NE |
|
|
Leads all day long 24 hours |
5 |
|
|
Only during the day |
4 |
|
|
Only during morning and evening |
3 |
|
|
Only during evening |
2 |
|
|
Attention assumed normalcy |
1 |
|
|
Orientation - (time, place, person, situation) |
Not afflicted in MS |
NAA |
|
Not existing |
NE |
|
|
No orientation for all 4 categories |
5 |
|
|
3 of the 4 categories affected |
4 |
|
|
2 of the 4 categories affected |
3 |
|
|
1 of the 4 categories (mostly time) affected |
2 |
|
|
Orientation assumed normalcy |
1 |
|
|
Depression |
Not afflicted in MS |
NAA |
|
Not existing |
NE |
|
|
Severe depression with suicidal tendencies |
5 |
|
|
Severe depression without suicidal tendencies |
4 |
|
|
Moderate depression |
3 |
|
|
Mild depression |
2 |
|
|
No depression |
1 |
|
|
Irritability |
Not afflicted in MS |
NAA |
|
Not existing |
NE |
|
|
Irritability 24 hours day and night |
5 |
|
|
Irritability all day long |
4 |
|
|
Irritability only during morning and evening |
3 |
|
|
Irritability only on walking up |
2 |
|
|
No Irritability |
1 |
|
|
Eye Pain |
Not afflicted in MS |
NAA |
|
Not existing |
NE |
|
|
Constant severe pain |
5 |
|
|
Intermittent severe pain |
4 |
|
|
Constant mild pain |
3 |
|
|
Intermittent mild pain |
2 |
|
|
Normal |
1 |
|
|
Floaters |
Not afflicted in MS |
NAA |
|
Not existing |
NE |
|
|
> 6 |
5 |
|
|
4 to 5 |
4 |
|
|
2 to 3 |
3 |
|
|
1 |
2 |
|
|
Normal |
1 |
|
|
Communication - Speech |
Not afflicted in MS |
NAA |
|
Not existing |
NE |
|
|
Depending on alternate communication system |
5 |
|
|
Disarticulated and cannot be understood |
4 |
|
|
Disarticulated but can be understood |
3 |
|
|
Slurred but still understandable |
2 |
|
|
Normal Speech |
1 |
|
|
Bowel - Sensation |
Not afflicted in MS |
NAA |
|
Not existing |
NE |
|
|
No bowel sensation |
5 |
|
|
Bowel Sensation restored |
1 |
|
|
Bowel - Control |
Not afflicted in MS |
NAA |
|
Not existing |
NE |
|
|
No bowel control |
5 |
|
|
Bowel control restored |
1 |
|
|
Bladder - Sensation |
Not afflicted in MS |
NAA |
|
Not existing |
NE |
|
|
No bladder sensation |
5 |
|
|
Sensation only at extreme filling >100 ml |
4 |
|
|
At 700 - 900 ml |
3 |
|
|
At 300 - 600 ml |
2 |
|
|
At less than 200 ml |
1 |
|
|
Bladder - Control |
Not afflicted in MS |
NAA |
|
Not existing |
NE |
|
|
No bladder Control |
5 |
|
|
Grossly impaired |
4 |
|
|
Incontinence in 1-3 times per week |
3 |
|
|
Incontinence 1 time per week |
2 |
|
|
Bladder controlled |
1 |
|
|
Sleep disorder |
|
|
|
Hypersomnia |
Not afflicted in MS |
NAA |
|
Not existing |
NE |
|
|
> 13 hours sleep per day |
5 |
|
|
11 to 13 hours sleep per day |
4 |
|
|
9 to 11 hours sleep per day |
3 |
|
|
8 to 9 hours sleep per day |
2 |
|
|
Hypersomnia disappeared |
1 |
|
|
Hyposomnia |
Not afflicted in MS |
NAA |
|
Not existing |
NE |
|
|
No sleep despite sleeping medicines |
5 |
|
|
< 4 hours sleep with medicines |
4 |
|
|
4 to 6 hours sleep with medicines |
3 |
|
|
6 to 8 hours sleep with medicine |
2 |
|
|
Hyposomnia disappeared, i.e. normal sleep with no medicines |
1 |
|
|
Appetite |
Not afflicted in MS |
NAA |
|
Not existing |
NE |
|
|
No appetite at all |
5 |
|
|
No appetite most of the day |
4 |
|
|
No appetite during first half of the day |
3 |
|
|
Appetite only sometimes |
2 |
|
|
Appetite assumed normalcy |
1 |
|
|
Breathing Pattern |
|
|
|
Bradycardia |
Not afflicted in MS |
NAA |
|
Not existing |
NE |
|
|
Bradycardia < 7 |
5 |
|
|
Bradycardia between 7 to 9 |
4 |
|
|
Bradycardia between 9 to 10 |
3 |
|
|
Bradycardia between 10 to 11 |
2 |
|
|
Bradycardia assumed normalcy |
1 |
|
|
Tachycardia |
Not afflicted in MS |
NAA |
|
Not existing |
NE |
|
|
Tachycardia>31 |
5 |
|
|
Tachycardia between 29 to 31 |
4 |
|
|
Tachycardia between 26 t0 28 |
3 |
|
|
Tachycardia between 23 to 25 |
2 |
|
|
Tachycardia assumed normalcy |
1 |
|
|
Physical - drooling |
Not afflicted in MS |
NAA |
|
Not existing |
NE |
|
|
Severe drooling |
5 |
|
|
Moderate drooling |
4 |
|
|
Mild drooling |
3 |
|
|
On and off |
2 |
|
|
Normal |
1 |
|
|
Deformity (Areas affected: back, right upper limbs, right lower limbs, left upper limbs, left lower limbs) |
Not afflicted in MS |
NAA |
|
Not existing |
NE |
|
|
More than 3 areas involved |
5 |
|
|
3 areas involved |
4 |
|
|
2 areas involved |
3 |
|
|
1 area involved |
2 |
|
|
Deformity disappeared |
1 |
|
|
Callipers |
Not afflicted in MS |
NAA |
|
Not existing |
NE |
|
|
THKAFO |
5 |
|
|
HKAFO |
4 |
|
|
KAFO + / Shannon brace |
3 |
|
|
AFO + / knee extension / Shannon brace |
2 |
|
|
AFO / Knee extension / Shannon brace |
1 |
|
|
Abbreviations: MS: Multiple Sclerosis; U/L: Upper Limb; Ml: Millilitre; THKAFO: Trunk Hip Knee Ankle Foot Orthosis; HKAFO: Hip Knee Ankle Foot Orthosis; KAFO: Knee Ankle Foot Orthosis; AFO: Ankle Foot Orthosis |
||
represents RNFS grades for all the parameters.
The term not afflicted in ailment (NAA) is used to grade a parameter that is not associated with the ailment. Not existing (NE) is used to grade a parameter for which a case is too young to respond to the diagnosis. RNFS covers various parameters which are essential for the evaluation of MS patients. (Figure 1)
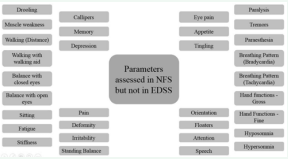
Figure 1 List of Parameters Assessed in Reverse Nutech Functional Score but not in Expanded Disability Status Scale
represents the list of parameters which are covered in RNFS but not in EDSS.
In RNFS, the five ordinal grades (1,2,3,4,5) run in the direction 1 → 5, i.e., GOOD → BAD. These five grades that are equidistant to each other and are continuous lie in a range of (0.5,5.5). To conduct probability based studies which requires a range of (-1,1) or (0,1), we have converted the grades into numeric values. This configuration can be used universally for any symptom. For converting categorical scores into numeric scores, a polynomial smoothing and graphical method has been used to derive an equation. The equation is as follows:
Yn = 0.096 × (Yc + 0.5) – 0.166
Where Yn = numeric score and Yc = categorical score
(Table 2) shows the conversion of five/ three categorical grades (0.5- 5.5) into five/ three numeric grades in the range (0,1).
|
Table 2: Conversion Table from Categorical Scores to Numeric Range for Reverse Nutech Functional Score. |
||||||
|
No. of scores |
Numeric (Yn) |
Categorical scores (Yc) |
||||
|
1 |
2 |
3 |
4 |
5 |
||
|
5 |
Score |
0.122 |
0.310 |
0.500 |
0.690 |
0.89 |
|
Range |
0-0.241 |
0.241-0.379 |
0.379-0.621 |
0.621-0.759 |
0.759-1.00 |
|
|
3 |
Score |
0.167 |
0.500 |
0.833 |
- |
- |
|
Range |
0-0.333 |
0.333-0.667 |
0.667-1.00 |
- |
- |
|
DISCUSSION
MS is an auto immune disease which can cause a variety of symptoms such as hypoesthesia, muscle weakness, dysarthria or dysphagia, nystagmus, abnormal muscle spasms, or difficulty in moving, coordination and balance; optic neuritis, phosphenes or diplopia, cognitive impairment, fatigue and acute or chronic pain syndromes, bladder and bowel difficulties, or emotional symptomatology [15]. Various diagnostic tests are used for the diagnosis of MS in patients, viz. magnetic resonance imaging (MRI), computed tomography (CT), cerebrospinal fluid analysis (CFA), and evoked potential testing [16].
Besides these diagnostic tests, a functional rating scale called EDSS, based on symptoms, is generally used to evaluate the patients with MS. EDSS evaluates eight FS that include pyramidal, cerebellar, brainstem, sensory, bladder and bowel, vision, cerebral and others. It has 10 grades or steps beyond 0 (normal), extending to status 10 (death due to MS). Patients are assigned severity score based on their neurologic examination that ranges from 0-10 in increments of 0.5 [2].
EDSS also has a number of limitations. It is dependent on mobility of a patient. It is subjective in certain areas (e.g., bowel and bladder function). It is also insensitive to minor changes in patient’s condition. Further, it does not present an accurate picture of the patient’s cognitive abilities and functional abilities in performing activities of daily living (ADL). It is non-linear in terms of the time spent at various ranges of the scale [17]. Other scoring systems such as FAMS also have some limitations. Bethoux et al., reported that FAMS was not found to be more responsive in walking based measures during psychometric validation [3].
To mitigate these limitations, a new scoring system has been introduced called RNFS. RNFS for MS is a 36-point positional and directional scoring system that can be used to assess a patient with MS or confirm the diagnosis of the patient with MS. EDSS appears to be a broad classification symptom as the difference in improvement is not clearly recognizable. But in RNFS, even the slightest improvement in the patient’s symptom is noted. Thus, evaluation of the patient’s condition using RNFS seems to be much more accurate. EDSS is mainly dependent on the mobility of the patient, whereas, in RNFS all the parameters including vision, paralysis, balance, pain and psychological parameters are evaluated.
The scoring system of RNFS is also easier and less tedious than EDSS. In RNFS, each parameter is scored from 1 → 5, i.e., GOOD → BAD depicting an improvement in patient. Though in EDSS, there is a broader classification of scoring; the scoring system is not uniform, as all the parameters are not assigned the similar pattern of scores/points for extreme as well as intermediate levels.
To further explain the usefulness of RNFS, let us take a hypothetical example of a MS patient assessed with both the scoring systems (EDSS and RNFS). The patient is evaluated for muscle weakness. As per RNFS, the muscle weakness is scored 1: if the patient is in normal condition; 2: if 1 area is involved; 3: if 2 areas are involved; 4: if 3 areas are involved; 5: if more than 3 areas are involved, and if the patient does not have muscle weakness then it is referred as NAA. Suppose the patient scored 4 for muscle weakness before the treatment. Similarly, all other symptoms have been scored with RNFS for this patient (Table 3).
|
Table 3: Example of Hypothetical Scores of Patient Before and After the Treatment. |
||||||
|
Parameters |
NFS Scores |
Parameters |
EDSS Scores |
|||
|
Before |
After |
|
Before |
After |
||
|
Muscle weakness |
4 |
2 |
Pyramidal |
8 |
4 |
|
|
Distance |
3 |
2 |
Cerebellar |
6 |
3 |
|
|
Walking Aid |
4 |
2 |
Brainstem |
7 |
5 |
|
|
Eyes closed in straight line |
NAA |
NAA |
Sensory |
9 |
4 |
|
|
Eyes open in straight line |
NAA |
NAA |
Bladder and bowel |
8 |
3 |
|
|
Sitting |
5 |
3 |
Vision |
6 |
4 |
|
|
Fatigue |
5 |
2 |
Cerebral |
7 |
3 |
|
|
Stiffness |
3 |
1 |
Other |
8 |
2 |
|
|
Tremors |
4 |
2 |
|
|
|
|
|
Paraesthesia |
5 |
3 |
|
|
|
|
|
Pain |
4 |
2 |
|
|
|
|
|
Hand functions - Gross |
NAA |
NAA |
|
|
|
|
|
Hand functions - Fine |
NAA |
NAA |
|
|
|
|
|
Standing balance |
4 |
3 |
|
|
|
|
|
Tingling |
5 |
2 |
|
|
|
|
|
Paralysis |
3 |
2 |
|
|
|
|
|
Memory |
4 |
2 |
|
|
|
|
|
Attention |
5 |
3 |
|
|
|
|
|
Orientation - Yes or No |
4 |
1 |
|
|
|
|
|
Depression |
3 |
1 |
|
|
|
|
|
Irritability |
3 |
1 |
|
|
|
|
|
Eye pain |
NAA |
NAA |
|
|
|
|
|
Floaters |
4 |
2 |
|
|
|
|
|
Communication - speech |
3 |
1 |
|
|
|
|
|
Bowel – sensation |
4 |
3 |
|
|
|
|
|
Bowel – control |
4 |
2 |
|
|
|
|
|
Bladder – sensation |
4 |
2 |
|
|
|
|
|
Bladder – control |
4 |
2 |
|
|
|
|
|
Hypersomnia |
3 |
1 |
|
|
|
|
|
Hyposomnia |
NAA |
NAA |
|
|
|
|
|
Appetite |
4 |
2 |
|
|
|
|
|
Breathing pattern – bradycardia |
NAA |
NAA |
|
|
|
|
|
Breathing pattern – tachycardia |
3 |
1 |
|
|
|
|
|
Drooling |
4 |
2 |
|
|
|
|
|
Deformity |
3 |
1 |
|
|
|
|
|
Callipers |
5 |
2 |
|
|
|
|
|
Total |
113 |
55 |
59 |
28 |
|
|
The total RNFS score for the patient is cumulative scores of all the symptoms. This patient is also scored as per EDSS simultaneously.
As per RNFS, the patient scored 113 and 55, before and after the treatment, respectively and as per EDSS, the patient scored 59 and 28, before and after the treatment, respectively (Table 3).
According to RNFS, a patient is considered normal if the total score is less than 36. The condition of patients is considered worst if the score falls in the range of 144 to 180 and bad if the score falls in the range of 72 to 108. In EDSS, the patient is scored on broader classification whereas, in RNFS, each parameter is further classified in five categories. The advantages of scoring system are that it depicts even the slightest improvement/ deterioration in symptoms of the patient by addition and subtraction of the scores. This in turn helps to decide the treatment strategy. However in EDSS, the patient is graded to be in a single level that describes his/her present functional abilities.
However, RNFS has only been developed and used at our facility. We encourage the use of this system worldwide by other physicians and healthcare professionals to assess it in a larger number of patients and different settings. This will help to assess the reliability of RNFS scoring system to a wider extent.
CONCLUSION
RNFS can be considered as a unique tool to assess the patients with MS. It is a functional and numeric scoring system which is easy to use. Even slight changes in the condition of patient can be noted with RNFS. Future studies from different settings in a larger number of patients will help in its acceptance and universal use.
ACKNOWLEDGEMENTS
The author acknowledges all the doctors, staff and patients of the Nutech Mediworld. The author also acknowledges Knowledge Isotopes Pvt. Ltd. (http://www.knowledgeisotopes.com) for the writing assistance.






































































































































