Stroke and Cancer- A Complicated Relationship
- 1. Department of Neurology, Johns Hopkins Hospital, Baltimore MD
Abstract
The interrelationship between stroke and cancer is complex. Cancer and stroke may occur independently in a given patient, or cancer may directly or indirectly lead to stroke via: hypercoaguability, non-bacterial thrombotic endocarditis (NBTE), direct tumor compression of blood vessels, or treatment-related effects which potentiate stroke. Patients with cryptogenic stroke are relatively common, and under the right circumstances, may provide an opportunity to screen for occult malignancy. In this review, we discuss relevant data linking stroke and cancer as well as propose a testable algorithm for cancer screening in the patient with cryptogenic stroke. Future directions should focus on validating patient-care algorithms in prospective clinical trials to provide an evidence base for this important issue.
Keywords
• Stroke
• Cancer
• Hypercoagulable States
• Venous Thromboemoblism
• Non- Bacterial Thrombotic Endocarditis
Citation
Dearborn JL, Urrutia VC, Zeiler SR (2014) Stroke and Cancer- A Complicated Relationship. J Neurol Transl Neurosci 2(1): 1039
INTRODUCTION
Cancer and ischemic stroke independently carry a large burden of morbidity and mortality as the second and fourth leading cause of death in the United States [1]. They each represent an enormous expenditure as a percentage of health care resources, manifested by lost productivity and disrupted family structures due to death and dependency. Cancer patients frequently have strokes, both from traditional risk factors and from mechanisms thought unique to malignancy. An autopsy study of patients with known cancer at time of death showed 15% of patients suffer from stroke diagnosed pathologically; [2] however only half of these strokes were noted during life. In addition, patients with venous thromboembolism are more likely to be diagnosed with cancer in the ensuing years, suggesting that hypercoaguability may be an important first presentation in cancer [3]. Patients with stroke and cancer have poorer clinical outcomes and longer hospital stays compared with stroke patients without cancer [4]. Unfortunately, detection, prevention, and treatment of stroke in cancer patients have been largely understudied. In addition, clinicians in many stroke centers must grapple with the fairly high rate of embolic-appearing stroke where no etiology is found. This rate of cryptogenic stroke has been quoted as high as 26-40 % of patients [5-8]. It is tempting to consider hypercoagulability from a previously undiagnosed cancer as a possible etiology in such patients [4,9]. In this review, we will focus on the data linking cancer as a possible etiology of stroke as well as suggest possible diagnostic and management schemes.
BACKGROUND
The most frequent causes of stroke in cancer patients are traditional cerebrovascular risk factors such as hypertension, hyperlipidemia, diabetes, atrial fibrillation and tobacco use [10- 12]. Vascular risk profiles of cancer patients are similar when compared to patients without cancer who were admitted to a stroke unit [13]. Nevertheless, the classification of “cryptogenic” stroke, meaning that no cause was identified despite detailed investigation, is more common in stroke patients with cancer, [10] suggesting an association between malignancy and unknown mechanisms leading to stroke. Of note, 67% of strokes in cancer patients appear as multiple embolic events on imaging in one study [14] suggesting that clot formation and embolization may often be the culprit. Although cancer can co-exist and even accelerate traditional cerebrovascular risk factors, we hypothesize that in a certain subset of patients, cancer causes stroke more directly. Multiple mechanisms, supported by multiple lines of evidence, may link stroke with cancer. We explore each below. Findings are summarized in Table 1.
Table 1: Cancer Related Mechanisms of Stroke.
|
Mechanism |
Causal factor |
Associated tumors |
Stroke Characteristics |
|
Hypercoagulability |
Adenocarcinomas especially; secrete mucin; tumors activate coagulation cascade; release pro-coagulant cytokines |
Adenocarcinoma of breast, lung, prostate, etc. Also brain, kidney or hematologic malignancies |
Embolic appearing infarcts, end vessels |
|
Venous-to-arterial embolism |
PFO, right-to-left shunt |
Uncertain, likely similar to tumors of hypercoagulable state |
Embolic appearing |
|
Non bacterial thrombotic endocarditis |
Sterile vegetations, clumps of platelets and fibrin develop on aortic valve |
Adenocarcinoma is most common |
Multiple widely distributed small and large strokes |
|
Direct tumor compression of vessel |
Tumor growth and resultant edema compresses major intracranial vessel |
Glioblastoma multiforme, metastasis to brain |
Large vessel, MCA common |
|
Tumor embolism |
Rare- cardiac tumor causes embolization of malignant cells |
Atrial or aortic valve myxoma, metastatic tumors to heart |
Embolic appearing |
|
Hyperviscocity |
Rare-“Thickened” blood causes hypoviscious obstruction of small end vessels |
Polycythemia vera, multiple myeloma, Waldenstrom’s Macroglobulinemia, leptomeningeal carcinomatosis |
Small end-vessels strokes |
|
Angioinvasive/infiltrative |
Rare-Hematologic malignancies infiltrate blood vessel wall, causing irregularities that predispose to arterial embolism |
B-cell lymphoma |
Multiple vascular territory infarcts |
|
Post –radiation vasculopathy |
Radiation after head and neck cancer causes vasculopathy leading to accelerated atherosclerosis, predisposing to vessel wall irregularities and embolism |
Squamous cell carcinoma, other head and neck tumors |
Embolic stroke from the affected carotid |
|
Chemotherapy associated |
Unknown |
Associated with as cisplatin, methotrexate, L-aspariginase, thalidomide, lenalidomide, and bevacizumab |
Varied |
Hypercoagulability
Perhaps the most important and underreported mechanism by which cancer can cause stroke is via abnormal coagulation cascades. The eponymous Trousseau’s syndrome, first described in 1865, referred to migratory thrombophlebitis in a patient with a visceral carcinoma, [15] but has since been expanded to describe any hypercoagulable state associated with cancer [16]. Coagulation disorders, such as disseminated intravascular coagulation (DIC), are more likely to be seen in stroke patients with cancer than without [4,11,12]. Cancer patients with cryptogenic stroke were found to have elevated D-Dimer levels compared to stroke patients without malignancy [9].
Although many malignancies have been associated with hypercoagulability, adenocarcinoma is frequently linked with clotting disorders as well as malignancy-associated stroke and, therefore, bears special consideration. In Japan, the incidence of colorectal cancer has been reported to be 16 per 10,000 person years for the general population; however, the incidence was found to be nearly 500 times in patients presenting initially with stroke [17]. Mechanistically, adenocarcinomas are thought to potentiate thrombi via production of mucin, a high molecular weight “sticky” molecule that is glycosylated and secreted normally by endothelial cells. Adenocarcinomas especially of the pancreas, colon, breast, lung, prostate, and ovary can secrete this molecule directly into the bloodstream, precipitating a viscous, and hypercoagulable state [15,18]. Mucin can interact with certain cell adhesion molecules (CAM), on endothelial cells, platelets, and lymphocytes to induce the formation of platelet rich microthrombi.
Further, tumor cells can release pro-coagulant molecules directly, the most well know of which are tissue factor (TF) and cancer pro-coagulant (CP) [19,20]. TF is a protein that binds to factor VII to potentiate the coagulation cascade, and thereby thrombosis. TF has been found in symptomatic atherosclerotic plaques in carotid stenosis, prompting the hypothesis that TF destabilizes plaque [21,22]. CP is released by the majority of cancers (81% in one sample assay), and is known to be a cysteine proteinase which directly cleaves factor X to Xa, ultimately resulting in the generation of thrombin [23,24].
Tumor-endothelial reactions are important for release of local chemical mediators. Malignant cells release pro-coagulant cytokines such as TNF-alpha, IL-1 and IL-6, causing sloughing of vascular endothelial cells as well as increased blood sludging [23,24]. These cytokines induce endothelial cells, monocytes, and cancer cells to express TF, thereby potentiating the clotting cascade [24]. In addition, these cytokines inhibit Protein C activation thus decreasing a natural “brake” on the anticoagulation system [20]. Platelet activation is also increased in cancer patients, likely from multiple mechanisms of locally released cytokines and secreted proteins by tumor and elevated levels of von-Willebrand factor [20].
Venous-to-arterial embolism
Perhaps the most well recognized clinical presentation of hypercoagulability is deep venous thrombosis and/or pulmonary embolus. These venous clots may lead to stroke via a direct venous-to-arterial shunting – sometimes referred to as “paradoxical” emboli. There is debate about whether venous to arterial thrombo-embolization via a patent foramen ovale (PFO) occurs. The likelihood of having a stroke in patients with PFO is doubled, suggesting that something about the shunt increases stroke risk [25]. On the other hand, neither the size of the PFO or the degree of venous-to-arterial shunting correlated with risk of stroke recurrence [26]. One interesting study found an increased rate of pelvic thrombosis in patients with cryptogenic stroke, some of whom had a PFO, suggesting a possible mechanism [27]. Nevertheless, it seems reasonable that increased risk of venous clots increases the risk of paradoxical embolization [28].
Nonbacterial thrombotic endocarditis
Another common mechanism relating stroke and cancer is nonbacterial thrombotic endocarditis (NBTE), previously known as marantic endocarditis. In NBTE, sterile vegetations develop on the cardiac valves, in descending order of frequency: aortic, mitral, and a combination of aortic and mitral [29]. The mechanism is thought to arise from disrupted fibrin attaching to previously undamaged valves in high flow areas and developing a network onto which platelets can adhere. Transesophageal echocardiography (TEE) is thought to be more sensitive that transthoracic echocardiography (TTE) in detecting valvular vegetations [30]. Often a TEE is not part of a standard stroke workup. In a retrospective study 8 of 24 patients with cancer were found to have NBTE, [4] which is frequently associated with adenocarcinoma [31]. Systemic emboli occur in nearly 50% of patients with NBTE, with cerebral emboli being quite common [29,32]. The diffusion MRI pattern in patients with NBTE was uniformly found to have multiple widely distributed small and large strokes, whereas those with bacterial endocarditis had more varied stroke patterns, sometimes involving a single vascular distribution [33].
Direct tumor effects
Direct tumor effects, either from tumor compression, or from tumor embolism are another cancer specific mechanism of stroke. Metastases to the brain, as well as primary brain tumors, can cause direct compression of blood vessels, either by direct tumor invasion or via tumor bed edema, [34-36] leading to cerebral ischemia and subsequent infarction in the territory distal to the affected vessel. This presentation can be difficult to clinically differentiate from tumor progression alone. It bears special mention that direct tumor effects can also lead to hemorrhagic stroke within the cranial vault. Hemorrhagic conversion of brain metastasis is a relatively rare occurrence in a population of non-hypertensive hemorrhagic strokes (1 to 10% of cases) [37]. Melanoma, renal cell carcinoma, and choriocarcinoma are some examples of tumor types with a tendency for hemorrhagic conversion based on case studies [37,38]. The mechanism is likely related to necrosis of tumor beds, which are rich in vasculature.
Other rare causes of direct cancer effects leading to stroke include embolism to the brain from metastasis in the heart [39-41]. Tumors that are most likely to affect the heart include melanoma, which has a high rate of hematologic spread, carcinomas of the lung, breast, esophagus or hematologic malignancies, although many tumor types have been reported [42,43]. Primary cardiac tumors, such as atrial myxomas, although benign also have embolic potential, [42,44] and hematologic malignancies can result in strokes by directly affecting intracranial structures and/or blood flow. Hyperviscous obstruction of end vessels from the malignant hematologic cells, as exemplified by polycythemia vera, can lead to decreased perfusion and stroke [45,46]. Intravascular lymphomatosis, also known as angiotropic lymphoma or neoplastic angioendotheliosis, is primarily of B-cell origin and can cause multiple territory cerebral infarcts by an infiltrative process. Other organs are typically spared, although skin involvement is not uncommon [36,47,48]. This is often an elusive diagnosis, as much of the testing for systemic disease can be negative.
Cancer-associated treatments and stroke
Radiation treatment effect causes stroke by unique mechanisms [49]. Head and neck radiation causes a vasculopathy of medium and large sized vessels that often presents years after radiation exposure. This vasculopathy is not well characterized, but may be associated with accelerated atherosclerosis [50-53]. Regardless of the underlying pathophysiology, the changes can lead to radiological findings similar to Moyamoya syndrome. Patients develop stenosis of the carotid vessel with abnormal netlike vessels and transdural anastomosis distal to the stenosis [54]. Head and neck radiation therapy (HNXRT) may almost double the risk of stroke, with the exception of adjuvant breast radiation therapy where neck exposure is minimal [55]. Squamous cell carcinoma is the most common cancer treated using HNXRT. In one analysis, the overall rate of stroke was 1.44 times higher in the radiation therapy cohort than the reference cohort [56].
Some chemotherapeutic agents have also been associated with an increased risk of stroke, such as cisplatin, methotrexate and L-aspariginase, however the mechanisms are poorly understood [57,58]. They are thought to be related to thromboembolic events (both venous and arterial). For example, L-aspariginase has been associated with cerebral venous thrombosis in children treated for leukemia [36]. The antiangiogenic agents thalidomide and lenalidomide have been associated with stroke [59-61] and are associated with a high risk of VTE [62,63].
Bevacizumab, a monoclonal antibody against VEG-F receptors is used in a variety of cancer types including glioblastoma multiforme and other solid tumors. It is associated with a 3% arterial thrombotic event rate, [62] however pooled analysis did not show an increased risk of VTE [64,65]. In treatment of solid tumors, including breast, colon and non-small cell lung cancer, bevacizumab plus chemotherapy, compared with chemotherapy alone, has a hazard ratio of 2.0 for arterial thrombotic events [65]. A retrospective analysis of the cohort with glioblastoma multiforme in treatment trials for bevacizumab showed a stroke rate of 1.9%, and a hemorrhagic stroke rate of 1.9%. The attributable risk from the drug rather than malignancy or traditional risk factors is unclear [66].
Screening for cancer in the patient with cryptogenic stroke
The data suggests that cancer is either directly or indirectly responsible for stroke in a certain subset of patients (as opposed to just coexistent with vascular risk factors). As such, clinicians should consider screening for occult cancer in a subset of cryptogenic stroke patients. However, the subset of patients requiring stroke-prompted cancer screening, the optimal diagnostic approach, and how the approach should differ based upon age, clinical presentation, and associated risk factors, has yet to be determined. Using existing data, we suggest an approach that may help to diagnose occult cancer as well as provide testable hypotheses for future improvement of this methodology in stroke patients with cryptogenic stroke (Figure 1).
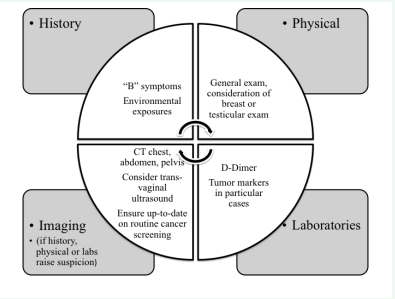
Figure 1 An approach for malignancy screening in patients with cryptogenic stroke. In this approach, every patient with an embolic appearing cryptogenic stroke (regardless of age), undergoes an expanded history, physical examination, and serological work-up (D-Dimer). Patients with one or more finding on history, physical examination, or serum testing suspicious for malignancy should undergo further evaluation with imaging
We focus on diagnostics associated with occult cancer-induced hypercoaguability, since other causes of cancer-induced stroke (e. g. direct tumor effects and treatment effects) are often apparent.
Our approach assumes that patients with an identifiable stroke etiology (e. g. atrial fibrillation) do not need stroke-prompted cancer screening, as the cause of stroke is already evident. Our approach includes stroke patients of all ages, as we freely admit that that the optimal age to screen for cancer in cryptogenic stroke patients is not known: older stroke patients are more likely to have cancer; [67] on the other hand, younger stroke patients are more likely to have a cryptogenic classification thus prompting further investigation [68].
We begin with imaging characteristics. Multiple embolic events (involving the brain and/or other organs) or presence of VTE, prompts a more extensive work-up. Generally, we do not consider lacunar stroke to be related to cancer-associated hypercoagulability since lacunar stroke occurs through different mechanisms [69,70]. When a cryptogenic stroke appears embolic, we expand the medical history to include symptoms suggestive of cancer (i. e. , the presence of “B” symptoms such as unexplained fevers, weight loss, and malaise). This should also include questioning for environmental exposures associated with cancer incidence (e. g. smoking and carcinogen exposures). We perform a careful general physical examination including a breast or testicular exam in the appropriate setting. Next, consideration is given to the evaluation of serum markers (such as D-Dimer) known to correlate with the diagnosis of cancer, which could help to raise or lower suspicion [71,72]. D-Dimer has an unknown sensitivity as a screening tool, but elevation in cancer patients with stroke is well documented [73-75]. The presence of one or more of these “red-flags” should prompt further workup: (1) contrast enhanced CT scanning of the chest, abdomen, and pelvis (PET scanning is a viable alternative), (2) ensuring that the patient is up-to-date on age appropriate cancer screening, and (3) consideration of trans-vaginal ultrasound in high risk women (age >50 years, age >25 with family history) [76] Testicular ultrasound is not an effective screen for testicular cancer in men [77].
Treatment of cancer-related stroke
In the patient with cancer and stroke, identification of stroke risk factors independent of cancer is of utmost importance. “Classic” cerebrovascular risk factors (hypertension, hyperlipidemia, diabetes mellitus, atrial fibrillation, carotid disease, and tobacco use) remain the leading etiologies of stroke and risk factor modification is therefore paramount. Atrial fibrillation should still be considered over hypercoaguability of cancer in patients with embolic appearing strokes, and, if discovered, anticoagulation initiated. Patients without a proven need for anticoagulation (eg. , hypercoaguable state such as Factor V Leiden disorder, large vessel dissection, atrial fibrillation, etc. ) should be started on an anti-platelet agent. Managing hypertension, hyperlipidemia, and diabetes; offering smoking cessation counseling; providing life-style modification counseling; and encouraging medication adherence are essential.
Patients with known cancer and probable cancer-related stroke bear special consideration. We should note at the outset that there are no direct studies that address treatment of any of the presumed cancer-induced stroke mechanisms discussed above. Of particular concern is prevention of hypercoaguability-induced stroke. Available data suggest hypercoaguability-induced stroke is a real entity, but difficult to diagnostically confirm or design a treatment plan effectively acknowledging the risks and benefits.
It remains to be seen whether there is a role for anti-platelets in the secondary prevention of cancer-related stroke (adenocarcinoma or otherwise). More data exist with respect to anticoagulation. One study measured the effect of anticoagulation (using unfractionated heparin, low molecular heparin, or warfarin) on micro-embolism [78]. Transcranial Doppler (TCD) was used in stroke patients with cancer to determine the embolic signal in the middle cerebral artery (MCA). Patients were divided into those with conventional stroke mechanism, and those with presumed hypercoagulability. Embolic signals measured by TCD were more commonly detected in patients with high D-Dimer levels. Treatment with anticoagulation was also noted to decrease D-Dimer levels [78]. This correlation between embolic signal and D-Dimer level may suggest that anticoagulation has the potential to attenuate cancer-induced hypercoaguability leading to stroke. Further, these data suggest that tracking D-Dimer levels may be a method to measure the risk of cancer-induced embolism and/or effect of anticoagulation in these patients.
Based upon these limited data, if we assume that anticoagulation is superior for the prevention of hypercoaguability-induced stroke, we must then consider which form of anticoagulation is most appropriate in cancer patients. There is data that low molecular weight heparin (LMWH) prevents VTE in cancer patients; however, whether the results can be extrapolated to arterial stroke is unclear. The results of trials with LMWH are summarized in Table 2.
Table 2: Important Studies of Venous Thromboembolism (VTE) and Cancer.
| Study | Type of study | Subjects | Intervention | Outcome |
| ENOXACAN II88 | Randomized, double blind, placebo controlled | 332 patients undergoing planned curative open surgery for abdominal or pelvic cancer | Enoxaparin vs placebo for 21 days. All patients received enoxaparin for first 6 to 10 days. | Reduced the incidence of VTE detected by ultrasound |
| CLOT79 | Randomized, open-label clinical trial | (N=336 treatment, n=336 standard care) Patients with cancer and a DVT, PE or both | Dalteparin for 5 or 6 days followed by Dalteparin vs warfarin for 6 months | Recurrent VTE lower in with no major increase in bleeding |
| Altinbas et al.89 | Randomized placebo controlled | 84 patients with squamous cell lung carcinoma receiving chemotherapy | Dalteparin vs placebo for 18 weeks | Dalteparin favorably improved survival |
| FAMOUS90 | Randomized placebo controlled | 385 patients with advanced malignancy | Dalteparin vs placebo for one year | Dalteparin did not improve survival |
| MALT91 | Randomized placebo controlled trial | (N=148 treatment, n=154 control) Patients with metastatic or locally advanced solid tumors | Nadroparin vs placebo for six weeks | Nadroparin favorably improved survival |
| LITE80 | Randomized, open-label clinical trial | (N=100 treatment, 100 standard care) Patients with cancer and a DVT, PE or both | Tinazeparin vs warfarin for 3 months | 3 month outcomes were similar, at 12 months VTE was reduced in the Tinazeparin group |
| PROSPECT92 | Phase IIb | 540 patients with locally advanced or metastatic pancreatic cancer undergoing chemotherapy | Enoxaparin vs placebo | Safety analysis showed no increase in bleeding in treatment group |
| PROTECHT82 | Randomized, double blind placebo controlled | (N=779 treatment and n=387 placebo) patients with metastatic or locally advanced solid cancer receiving chemotherapy | Nadroparin vs placebo for duration of chemotherapy versus 4 months | Reduced rate of stroke in nadroparin group, but only six total strokes occurred, also was increase in bleeding complications |
| SAVE-ONCO81 | Randomized, double blind placebo controlled | (N=1608 treatment, n=1604 placebo) Patients with metastatic or locally advanced cancer receiving chemotherapy | Semuloparin vs placebo until chemotherapy regimen was changed | Semuloparin reduced the risk of VTE, without an apparent increased risk of bleeding |
The CLOT study established the use of LMWH in patients with cancer and DVT. The probability of recurrent VTE was much lower in the dalteparin group at six months versus those treated with warfarin [79]. Similar results were seen with tinzaparin and semuloparin [80,81]. Enoxaparin has not been shown to be superior to warfarin, although the studies were often small and may not have been adequately powered [62]. A study evaluating antithrombotic prophylaxis with nadroparin versus placebo did include a subgroup analysis of patients with stroke [82]. Although there was a reduced rate of stroke in the nadroparin group, (3 of 769 patients) versus placebo group (3 of 381 patients) the total number of strokes was too small to draw concrete conclusions. The newer oral anticoagulants dabigatran (a direct thrombin inhibitor) and rivaroxiban (a direct factor Xa inhibitor), have proven effective in DVT treatment, [83] but remain to be studied in cancer subgroups.
Though there may be benefit to anticoagulation for stroke prevention in patients with cancer, there is also inherent risk. Large clinical trials show that systemic anticoagulation increases the rate of hemorrhage in ischemic stroke patients [84,85]. Developing a method for the selection of high-risk patients who may benefit the most using clinical (stroke severity), serologic (D-Dimer), and radiographic characteristics (presence of prior stroke), may be the optimal approach to deciding in whom to initiate therapy. In the VTE literature, there is a risk score for cancer patients used to guide which patients should be anticoagulated for primary prevention of DVT/VTE [86]. The score includes high-risk characteristics such as: type of cancer, platelet count, leukocyte count, D-Dimer, body mass index (BMI) and P-selectin. P-selectin is an adhesion molecule on endothelial cells and platelets that was independently shown to be a predictor of cancer associated VTE [87].
We suggest that patients with known cancer and cryptogenic, multiple embolic events (involving the brain and/or other organs), VTE, or the presence of adenocarcinoma represent a group with predisposition to hypercoaguability. (Generally, we do not consider lacunar stroke to be related to cancer-associated hypercoagulability since lacunar stroke occurs through different mechanisms. ) [69,70] If any of the above conditions are present, in the absence of another obvious cause for embolic stroke, patients are likely to benefit from anticoagulation with LMWH. In a patient in whom we are uncertain about the contribution of the cancer, we will often perform a TEE to investigate for NBTE. If present, NBTE would bias towards anticoagulation with LMWH. The TEE with bubble study can also tell us about the presence of a PFO, which by itself would not require anticoagulation, unless a DVT was present. Anticoagulation should likely be continued until the cancer is in remission, the patient cannot tolerate it, or a bleeding complication occurs.
CONCLUSIONS/FUTURE DIRECTIONS
Stroke and cancer are intertwined by virtue of their relatively common occurrence within the general population. It cannot be stressed enough that cancer is not the most common etiology of stroke, and that even in patients with cancer; the traditional risk factors are most commonly the underlying cause. There are, however, cancer-specific mechanisms that may further increase one’s risk for stroke. These include: hypercoagulable states induced by tumor cells, NBTE, vessel compression, chemotherapy related hypercoagulability or post radiation effects, hyperviscocity, or vessel infiltration by cancer cells. To date, there are no clinical trials leading to guidelines for diagnosis or treatment of these conditions. The DVT/VTE literature provides some insight into treatment of a subgroup of “hypercoagulable” stroke patients who may benefit from treatment from LMWH, but specific stroke prevention studies have yet to be performed.
Occult cancer may be an important missed diagnosis in cryptogenic stroke. There is little data to suggest what should be done to screen for malignancy in patients with cryptogenic stroke. First steps may include: 1) identifying a subgroup of stroke patients who are most likely to benefit from aggressive malignancy surveillance, and 2) subsequently carrying out this screening as part of the inpatient evaluation. Early screening will allow for more rapid detection of potentially treatable cancers, and may inform decisions on treatment for secondary stroke prevention. Our suggested approach needs to be validated in systematic studies to determine which patients require more aggressive screening and what are the most accurate diagnostic tests. Our approach also requires an analysis of the cost benefit of such a workup, as admittedly only 3% of all strokes are attributable to cancer and many screening tests such at PET are resource intensive and costly [17].
Finally, treatment of a patient with stroke in the setting of cancer is complex and requires careful integration from both neurologic and oncologic experts. Although existing data suggests LMWH may be superior, the role for antiplatelet agents versus anticoagulation with warfarin versus anticoagulation with LMWH is still unclear and requires further study. Treatment with chemotherapy and radiation also requires careful consideration when deciding how to proceed with cancer therapy post-stroke. An interdisciplinary team, including physician experts, therapists, and social workers, is best equipped to deal with the treatment decisions that follow.
REFERENCES
1. http://www.cdc.gov/nchs/fastats/lcod.htm
3. Prandoni P, Falanga A, Piccioli A. Cancer and venous thromboembolism. Lancet Oncol. 2005; 6: 401-410.
23. Bick RL. Cancer-associated thrombosis. N Engl J Med. 2003; 349: 109- 111.
36. Rogers LR. Cerebrovascular complications in patients with cancer. Semin Neurol. 2010; 30: 311-319.
37. Kase CS. Intracerebral hemorrhage: non-hypertensive causes. Stroke. 1986; 17: 590-595.
38. Mandybur TI. Intracranial hemorrhage caused by metastatic tumors. Neurology. 1977; 27: 650-655.
43. Reynen K, Köckeritz U, Strasser RH. Metastases to the heart. Ann Oncol. 2004; 15: 375-381.
76. Ilic D, Misso ML. Screening for testicular cancer. Cochrane Database Syst Rev. 2011; : CD007853.








































































































































