What Immunopathogenic Similarities Exist Between SARSCov-2 Infection And Multiple Sclerosis?: A Plea For Daily Vitamin D Supplementation To Improve Quality Of Life!
- 1. Independent researcher, Internal medicine, rehabilitation, social medicine Hochwaldstrasse 2 D-97769 Bad Brückenau, Germany
Abstract
For decades, arguments have been made for vitamin D supplementation as an add-on therapy “for” and “against” for people with multiple sclerosis. The COVID-19 pandemic has challenged therapists to protect high-risk groups, such as people with multiple sclerosis, from further health damage. In addition to the risk factors established in the general population, such as older age, male gender, hypertension, endocrinological and cardiovascular diseases, advanced disability, highly active disease with frequent drug therapies and doctor consultations as well as comorbidities accumulate in MS.
COVID-19 and MS have overlapping clinical and immunopathogenic properties. Due to the immunomodulatory properties of VitD, adjunctive therapy is likely to be consistent when COVID-19 occurs in PwMS.
Keywords
• Multiple sclerosis
• Immunopathogenesis
• SARS-CoV-2 infection
• Ebstein-Barr virus
• Vitamin D supplementation
• Ocrelizumab
CITATION
Goischke HK (2023) What Immunopathogenic Similarities Exist Between SARS-Cov-2 Infection And Multiple Sclerosis?: A Plea For Daily Vitamin D Supplementation To Improve Quality Of Life!. J Neurol Transl Neurosci 8(1): 1093.
INTRODUCTION
Multiple sclerosis (MS) is an inflammatory neurodegenerative disease with a suspected autoimmune origin. The disease begins earlier than current diagnostic criteria can detect. It affects the entire central nervous system and not just the white matter, as the original term – inflammatory demyelinating disorder suggests [1]. It is characterized by a very heterogeneous course of the disease, which is represented by relapse-associated neurological deterioration, but also by an increase in disability that is independent of relapse [2]. MS is associated with reduced vitamin D status [3].
Vitamin D (VitD) (1, 25(OH) 2D3) a neurosteroid, is known to influence brain development, neutransmission, and to exert neuroprotective and immunomodulatory functions [4,5].
The major form of vitamin D (Vit D), 25(OH) D and its active hormonal form, 1, 25-dihydroxy-vitamin D3, are present in the brain [6-9].
VitD signal transduction occurs via the vitamin D receptor (VDR) [5]. VDR is a part of the nuclear receptor superfamily of transcription factors [4]. Translocation of VDR to the nucleus is enhanced by calcitriol [10].
The VDR is expressed in almost all cell types of the body and various brain regions as well as in immune cells [11,12]. VDR is present in numerous brain cells such as oligodendrocytes, astrocytes, microglia and neurons [6]. The enzymes that convert 25(OH) D to 1, 25(OH) 2D3 (CYP27B1) and the enzymes responsible for its degradation (CYP24A1) have been detected by immunohistochemical methods [4]. In the human brain, VDR have been found in the hippocampus, hypothalamus, amygdala, cortex, cerebellum and substantia nigra, [6,12,13].
Although the cause of multiple sclerosis (MS) is not yet fully understood , interactions between B and T cells are discussed in the pathogenesis , such as peripheral escape of B cells from T cell-mediated control , interaction of pathogenic B and T cells in secondary lymph nodes and reactivation of B and T cells accumulating in the CNS (details in [14]. Auto reactive inflammatory cells , including effector T cells (Th1, Th17, CD8 + cytotoxic T cells), activated B cells and plasma cells producing autoantibodies infiltrates the central nervous system (CNS) [15].
This T cell migration into the CNS is the key to the pathogenesis of MS. The Treg cells (CD4+CD25+Foxp3 regulatory cells [Treg]) play an essential role in this. A dysfunction of both the Treg cells and the Tr1 (IL-10-secreting regulatory type 1 cells) has been confirmed [16,17]. Subsets of B cells function as antigen presenting cells and pro- inflammatory cytokine producing cells [18].
A supplementary supportive therapy to improve the course of the disease in people with multiple sclerosis (PwMS) is daily vitamin D supplementation with the aim of achieving s25(OH)D values of at least 60-80ng/ mL (30-130ng/ mL ) [19,20].
The effect of vitamin D (VitD) on the innate and acquired immune system is undisputed and 1, 25-dihydroxyvitamin D plays a major role in cell homeostasis in the CNS. 1, 25(OH) 2 D3 is autocrine and paracrine [21].
1, 25(OH) 2D3 also targets macrophages, monocytes, dendritic cells (DC), and T and B cells. VitD regulates the activation of microglia and astrocytes [22,23].
In DC, it decreases IL-12, TNF alpha and IL-6 production and promotes IL-10 production. The active metabolites of VitD also promotes the development of forkhead box protein 3 (FOXP3+ regulatory T (Treg) cells and IL-10-producing T regulatory type 1 (TR1) cells. 1, 25(OH) 2D3 blocks B- cell proliferation, plasma cell differentiation and immunoglobulin production [24-26].
Vitamin D exerts its complex immunomodulatory effects on CD4+ T lymphocytes (Th1, Th2, Th17, Treg cells), among others, and the secret of pro- inflammatory cytokines (IL-2, IL-6, IL-12, IL- 17, IFN-γ, TNF-α and TNF-β) is inhibited. It stimulates the production of anti- inflammatory regulatory Th2 cytokines (IL-4, IL-5 and IL-10) [26-29].
The cytokine profiles is shifted from a Th1 to a Th2 mediated profile (decrease in IFN-gamma and increase in IL-4 [28]. As a result, vitamin D insufficiency in PwMS must be avoided at all costs [28,30].
Not only is vit D deficiency associated with MS risk, but s25 (OH) D levels are inversely correlated with risk of relapse, CNS lesions, and disability progression. Vit D suppl. reduces the number of new Gd +- enhancing or new / enlarged T2 lesions on MRI [28-31].
COVID-19 infection
Severe acute respiratory syndrome coronavirus 2 (SARS CoV-2) is an exceptionally transmissible and pathogenic coronavirus that emerged in late 2019 and caused an acute respiratory disease pandemic known as coronavirus infection 2019 (COVID-19). New omicron variants are constantly being discovered [32].
COVID-19 can develop into a severe disease associated with immediate and delayed sequelae in various organs, including the central nervous system (CNS) [32].
Over the last 3 years, a complex connection between SARS CoV-2 infection and MS has emerged [33].
The renewed risk of a serious infection from COVID-19 should be an additional motivation for daily high-dose vitamin D administration [34,35]. In the context of prevention, it is worth mentioning that with circulating s25 (OH) D values ≥ 55ng/ mL, the SARS-CoV-2 positivity rate was significantly lower than with values below or with deficiency [36]. Current data suggest a protective role of VitD, particularly with a lower risk of intensive care unit admission and a reduced risk of death [37, 38]. In addition, the occurrence of long-COVID is an aspect of implementing this simple, effective, safe and cost-effective therapy with a broad therapeutic window for the prevention and treatment of COVID-19 disease [39-41].
Although there is still no indisputable evidence that Vit D supplementation (VitD suppl.) reduces the risk of SARS-Cov-2 infection in healthy people, but there is collective evidence that at-risk people have a benefit [42]. PwMS with comorbidities, psychiatric illnesses, hypertension, obesity (an increased BMI may correlate with a severe course of Covid-19), age > 50 years, severe disability and methylprednisolone boost therapy as well as some DMTs (disease- modifying therapies ) have a higher risk of infection and an increased risk of severe COVID-19 courses [43-45]. Infections (SARS-COV-2) can increase MS symptoms (pseudo-relapses) or cause real relapses [46]. In post-COVID syndrome (long COVID), one in eight patients presents with symptoms such as fatigue, shortness of breath, cough, joint pain, chest pain, muscle pain, headache and paresthesia in the limbs after at least 3 months. The latter can also occur in PwMS per se [47,48].
Mechanisms of action of Vit D on Covid-19
There have been hundreds of publications on this in the years since the outbreak of the COVID-19 infection. If people at risk have VitD supply. There is a lower risk of infection, the severity of the disease with admission to the intensive care unit or a reduced risk of death, Long Covid occurs less frequently [37-41,49-52]. It is not ethically justifiable to withhold a high dose of VitD from people at risk.
Barrea et al. list in detail 14 mechanisms as described by Vit D supply. The risk of COVID-19 infection can be reduced and sufficient Covid 19 vaccination is supported [48,53-58]. Vit D and its metabolites inactivate viruses (increase the antimicrobial peptide cathelicidin , lead to reduction of the risk of a cytokine storm , reduce matrix metalloproteinase-9 concentration and thereby increase the host’s metabolic tolerance to damage, reduce the risk of pneumonia and myocarditis, lead to the reduction of the concentration of pro-inflammatory cytokines, in particular interleukin 6 (IL-6), which promotes the permeability of the BBB, which leads to the potentiation of CNS damage in PwMS, is serious [48,60- 62].
Vit D enables neuroprotection by reducing inflammation and oxidative stress. Low 25(OH) D levels were inversely correlated with high IL-6 levels and were independent predictors of COVID-19 severity and mortality [63]. 1, 25(OH) 2D3 inhibits immunoglobulin synthesis, regulates B cell activity and reduces auto-Ab production. It converts B cells into plasma cells [37]. VitD reduces the risk of infection with EBV [48].
Long COVID- 19, Epstein-Barr virus (EBV), MS and inflammation
Current studies show evidence that the chronic inflammation in long Covid-19 infection with a reactivation of the latent Epstein-Barr virus (EBV) can lead to a worsening of the health status in PwMS [64-68]. In MS, there is a high affinity molecular mimicry between the EBV transcription factor EBNA-1 and the CNS protein GlialCAM (glial cell adhesion molecule of the central nervous system [69]. Bernal et al were able to detect EBV reactivation by detecting EBV DNA and antibodies against EBV lytic Genes provide [70].
In 66.7% of Long Covid patients, EBV reactivation could be demonstrated by a positive titer for EBV EA-D (early antigen- diffuse). IgG or EBV-VCA (viral capsid antigen)-IgM) can be provided [65]. Long COVID patients with fatigue and neurocognitive disorders were associated with serological evidence of recent EBV reactivation (early antigen D [EA-D] IgG positivity) or high nuclear antigen IgG levels [71].
The triad of inflammatory markers IL-1ß, IL-6 and TNF can be found in both Long Covid and MS [72-74]. Low sun exposure acts synergistically with high EBNA-1 Ab levels and was associated with an increased risk of MS [75].
There is a connection between high EBNA-1 antibody levels and low s25 (OH) D levels. On the other hand, a high dose of VitD lowers. The EBNA-1 antibody levels in PwMS [76-78]. Another parallel arises from the increase in GFAP (glial fibrillary acidic protein) as a functional disorder of the astrocytes around 4 months after the start of SARS-Cov2 infection [71]. The concentration of NfL (Neurofilament light chain), GFAP and total tau in CSF in patients with COVID-19 was often elevated with neurological symptoms [79]. Elevated sNfL has already been verified in mild to moderate COVID-19 disease [80]. On the other hand, the risk of mortality increased if sNfL and sGFAP levels were already elevated upon hospital admission [81].
Because there are no effective drugs that block EBV reactivation in Long Covid [82], there are multiple arguments for Vit D suppl. High-dose Vit D- Suppl (14,000IU/ day) for 48 weeks or 20,000 IU/week for 48 weeks selectively reduced anti-EBNA-1 antibody levels in PwMS (RRMS) [78,83].
Several mechanisms are under discussion: 1. VitD could induce better clearance of EBV-infected B cells, 2. Vit D could directly target and impair viral replication in EBV-infected cells, 3. Produce better control of inflammation in general, 4 In an EBV mediated inflammatory cascade, 1,25(OH)D3 could suppress the activation of reactive astrocytes 5. It is likely that at high s25 (OH) D levels, the VitD receptor EBNA 2 (Epstein -Barr virus nuclear antigen 2) is displaced upon DNA binding [78,83-88].
Early start of therapy is a crucial factor
The early start of therapy for VitD suppl. Is crucial in influencing influenza and COVID-19 infections [89].
The Corona-19 mortality risk therefore correlates inversely with the VitD status and a mortality rate close to zero could theoretically be achieved at over 50ng/ mL s25(OH)D [90]. The importance of Vit-D metabolism as a potential prophylactic, immunoregulatory and neuroprotective treatment of infections (COVID-19) is increasingly being considered in clinical practice as part of a multitherapeutic approach [91-93].
Dosage suggestions for vitamin D supplementation
Currently, there are no consensus guidelines suggesting an appropriate concentration of serum 25(OH) D to prevent COVID-19 or reduce its morbidity and mortality.
It is becoming increasingly clear to start with a “ loading dose ” with high doses of VitD over a few days and then continue with a “maintenance dose”, although various variants have been put up for discussion.
For example, one study used a weekly or fortnightly dose totaling 100,000–200,000 IU over 8 weeks (1800 or 3600 IU/ day) [94]. To obtain 75nmol/l s25 (OH) D values, the following equation was described:
Dose (IU) = 40 x (75 – serum 25(OH) D (3) [nmo /L] x body weight [94].
Over 30ng/ mL s25 (OH) D values were also achieved with a single oral dose of 200,000–600,000 IU [58, 95].
An s25 (OH) D level of 40-60ng/ml could be achieved by dosing up to 6000IU/day for several weeks [19,96]. A daily VitD intake of 10,000IU/day for 4 weeks would lead to a faster optimal s25 (OH) D level in the “status nascendi” of an infection [38].
Another dosage regimen was recommended: cholecalciferol 0.532 mg on day 1 and continued with 0.266 mg on days 3,7,14,21,28 (1 IU vitamin D3 ? 0.025 µ g vitamin D3 ? 65.0 mmol vitamin D3 [97]. The pharmacokinetic properties of calcifediol allow rapid absorption within hours, facilitating the immediate availability of 25OHD3 in the target tissue. This has drastically reduced the need for intensive care unit admission and the mortality rate [97]. Continued daily VitD dose will depend on the s25 (OH) values. The “maintenance dose” depends very individually on the achieved 25(OH) D serum levels and could be 4000-5000 IU/day (dose-response effect) [98].
Serum D level >50 ng / mL with a daily intake of 5000 IU (4000-7000 IU) and with obesity , gastrointestinal absorption disorders, antiepileptic or retroviral therapy, a two to four times higher dosage of VitD is required [99].
Daily doses of vitamin D have a therapeutic advantage over bolus doses
Reducing pro-inflammatory cytokines requires daily VitD administration because the cytokines have a short half-life of a few minutes to hours and are continuously produced by immune cells to remain elevated [100-102]. A daily VitD administration results in a better overall protective effect on general respiratory tract infections than a bolus administration [103].
Tores et al. was able to detect an increase in the anti inflammatory cytokine IL-10 in COVID-19 infection by administering 10,000IU/day for 14 days and achieve a shorter hospital stay compared to those patients who only received 2000IU/day [104]. Why very high doses of VitD supplementation may be necessary also arises from the knowledge that the SARS Cov-2 virus downregulates the VitD receptors, so that only high doses can still have an effect [48]. [Barrera]. Furthermore, VitD modulates ACE2 (Angiotensin Converting Enzyme 2) and can thereby cause ARDS (Acute Respiratory Distress syndrome) and alleviate acute lung damage caused by SARS-Cov-2 [105,106].
The dosage depends on the time and severity of the illness
However, for severe respiratory infections (COVID-19) or sepsis, a single oral bolus dose (100,000 to 500,000 IU) or in a divided dose of several times 50,000 IU is administered to ensure a rapid, adequate intracellular supply of calcitrol within 3 to 5 days and to make it available [107]. The COVIT-TRIAL study showed that in at-risk elderly patients, early administration of high-dose VitD in a single dose of 400,000IU within 72 hours of diagnosis improved all-cause mortality on day 14 compared to a standard dose of 50,000IU [49] . Short-term cholecalciferol administration with 60,000 IU over 7 days led to negative SARS-Cov-2 RNA in 41.7% of patients with mild symptoms or asymptomatic [108].
DISCUSSION
Both diseases, COVID-19 and MS, are associated with dysregulation of the immune system and mutually affect the course of the disease (pathophysiological/immunological mechanisms in detail in [32, 109]. The occurrence and severity of Covid-19 disease are associated with a worsening of clinical disability PwMS is linked, with several hypotheses currently being discussed. Details in [110]. In a prospective, ongoing MRI study (3 Tesla) (Post-Hospitalization COVID-19 Study) over 5-6 months, a reduction in the volume of the gray area was shown substance, white matter hyperintensities and vascular injury patterns. These qualitative MRI abnormalities were observed in 50% of post-COVID patients [111].
Elevated CRP (≥5mg/ mL) was associated with regional brain atrophy, requiring preventive measures through VitD suppl. challenges [112-114].
Low 25(OH)D levels are associated with a higher risk of infection, COVID-19 severity, respiratory distress severity, length of hospital stay, and mortality, and acute sequelae (long COVID) occur more frequently [92,115-119].
Before the previous consistent evidence of a connection between low s25(OH) values and negative SARS-Cov2 results in the general population is confirmed in high-quality, randomized studies [36], the administration of Vit D should be used for pathobiological reasons in PwMS .
The COVID-19 infection reinforces the need for a concept of a strategic MS treatment approach using all available therapies based on scientific knowledge and current experience [120].
Since the beginning of the coronavirus disease (COVID- 19) pandemic, different national recommendations for relapse therapy in RRMS with glucocorticoids as well as risk assessment statements have been issued for the management of PwMS [121,122]. And an international consensus Statement on care in the pandemic and post-pandemic period published in 2021 [123].
In the following years it became clear that patient-related data, such as increasing age, higher BMI, male gender, activity and type of MS subtypes, higher disability (higher EDSS), type of drug therapy, such as anti-CD -20 DMTs ( Ocrelizumab ) play a relevant role in optimizing and minimizing risk in the care of PwMS [124-126]. Janzuel et al reported that the use of anti CD-20 therapies in PwMS and RRMS was associated with severe COVID-19 courses [127]. On the other hand, it has been shown that for younger, more often female people with active disease and further therapies, the risk of infection increased through contact with doctors and medical facilities [128].
Vitamin D - a modifiable parameter in COVID-19 infection
It is undisputed in the literature that vitamin D exerts immunoregulatory properties on both the innate and the adaptive immune system and its deficiency is associated with increased autoimmunity and susceptibility to infections [28,32,96,129,130]. If in the general population a high s25(OH)D level (>30mg/ mL ) is associated with a lower risk of SARS-CoV-2 infection and severe COVID-19 progression as well as lower mortality compared to people with VitD insufficiency [129,131], daily VitD is recommended for PwMS suppl. On [48,96,118,131 136].
If the s25(OH)D value is recognized as a modifying factor for the positive influence of COVID-19 and LONG-COVID in PwMS , appropriate information must be given to this vulnerable group of people [99]. Those PwMS who have undergone anti-CD-20 therapy require special attention because severe COVID-19 courses are to be expected.
PwMS and ocrelizumab therapy showed breakthrough infection rates of 59% after the third SARS-CoV-2 mRNA vaccination [137]. Prophylactic VitD administration would therefore be particularly recommended to protect PwMS from infections. Although there are uncertainties regarding the optimal daily dosage of VitD (dose-response relationship taking body weight into account), the negative consequences could be reduced by pushing back the global “pandemic of VitD insufficiency/deficiency” [138-141]. In the era of COVID-19 infection and long-COVID [118,142-148].
COVID-19 and the endocrine system
SARS-CoV-2 penetrates the host cell primarily via ACE2 and TMPRSS2 (Tran’s membrane protease Serine2). These major receptors are also expressed in endocrine organs (thyroid, adrenal, hypothalamus, pituitary, gonads, and pancreas) [149,150]. During an infection of the endocrine system, the SARS-CoV-2 virus RNA has been detected in all endocrine organs, including the thyroid [149,151]. SARS-Cov-2 antigen was confirmed histopathologically in the thyroid gland postmortem [152].
Interactions between SARS-CoV-2 infection and the thyroid were confirmed by the description of “SARS-CoV-2-associated thyroiditis” in patients with COVID-19 [153]. An increase in thyroid autoantibodies TPOAb (thyroid peroxidase antibody) and TgAb (thyroglobulin-antibody) were observed during and after Covid-19 infection [154,155]. The prevalence of autoimmune thyroid diseases in the post-Covid phase was doubled compared to a control group [155]. People with a pre-existing autoimmune disease and Covid-19 were 23% more likely to be diagnosed with another autoimmune disease [156].
PwMS are affected by an increased manifestation of a coexisting autoimmune thyroid disease, especially Hashimoto thyroiditis [157]. This group of people with comorbidities such as Hashimoto’s thyroiditis or Graves’ disease should therefore be monitored endocrinologically both during the acute SARS CoV-2 infection and in the post-acute phase. Thyroid dysfunction is the most common comorbidity during therapy with DMT alemtuzumab [158].
In long-term follow-up, new symptoms that arise could easily be identified as being caused by endocrine disorders [159]. The exact mechanisms are not yet fully understood [45,150]. VitD deficiency could promote the autoimmune process [160,161]. A VitD supply. Lowers the serum TPO-Ab and TgAb titers and therefore this add-on therapy should also be used for prevention, especially when the endocrine system is involved [143,150,162 165].
Gut-microbiota-brain axis
Overlaps between Covid-19 and MS can be seen in the involvement of the gut-microbiota-brain axis. Dysfunctionality is characterized by changes in the bacterial composition and diversity of the microbiota [29,32,166-168]. A high-dose VitD supply. Could provide a benefit [41,168-170].
CONCLUSION
The clinical use of vitamin D supplementation will play an important role in the prevention and control of COVID-19 disease. Most research proved that vitamin D supplementation would bring clinical benefits to COVID-19 patients in terms of prevention and treatment (hospital length, mortality, improvement in blood oxygen saturation, better prognosis) [171].
There are no specific recommendations for everyday practice in connection with COVID-19 and vitamin D supplementation in patients with multiple sclerosis, but the scientific community also regrets that despite numerous “pro” and “against” voices, no consensus has been reached [172]. New corona variants will emerge, and, for ethical reasons, it is not justifiable to wait many years for the results of ongoing large-scale randomized studies [143] and their implementation in practice will certainly be delayed for years. Perhaps the idea is widely held that in a non existent ideal world all health decisions can only be made on the basis of overwhelming evidence, but a time of crisis may require slightly different rules [172].
The fight against SARS-CoV-2 is far from over because further virus variants will emerge. Eliminating vitamin D deficiency quickly is easy, inexpensive and quick to do. Depending on the course of the disease, comorbidities, treatment with disease modifying therapies and other diverse factors, multiple sclerosis is associated with an increased risk of a possible severe course of the COVID-19 infection. Vitamin D supplementation to prevent and influence the course of a COVID-19 infection in PwMS is presented and the supplementation protocols used to date are listed from the literature.
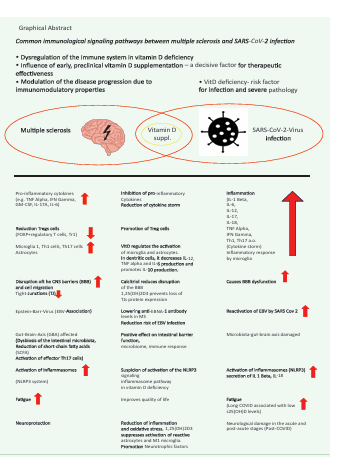
Graphical Abstract
REFERENCES
6. Eyles DW. Vitamin D: Brain and Behavior. JBMR Plus. 2020; 5: e10419.
evidence from Meta-Analysis and Trial Sequential Analysis. Pharmaceuticals (Basel). 2023; 16: 130.
89. Malaguarnera L. Vitamin D3 as Potential Treatment Adjuncts for COVID-19. Nutrients. 2020; 12: 3512.
107. Wimalawansa SJ. Physiological basis for Using Vitamin D to Improve health. Biomedicines. 2023; 11: 1542.
130. Aranow C. Vitamin D and the immune system. J Investig Med. 2011; 59: 881-886.
131. Sooriyaarachchi P, Jeyakumar DT, King N, Jayawardena R. Impact of vitamin D deficiency on COVID-19. Clin Nutr ASPEN. 2021; 44: 372 -378.
139. Holick MF. The vitamin D deficiency pandemic: Approaches for diagnosis, treatment and prevention. Rev Endocrin Metab Disord. 2017; 18:153-165.
153. Zetinig G. Thyroid and SARS-CoV- 2. J Klin EndoKrinol Stoffwechs. 2022; 15: 100-104.
158. Goischke HK. Alemtuzumab treatment-induced thyroid dysfunction in RRMS: a varied clinical picture in an interdisciplinary terrain. Act Neurol. 2017; 44: 1-8.
169. Bhargava P, Sotircho E, Eckstein Ch , Ntranos A, Gocke A, Mowry E et al. High dose vitamin D supplementation reduces IL-17-producing CD4+ cells and effector -memory CD+ T- cells in multiple sclerosis patients. Neurology. 2015: 84.