Alcohol, Cannabis and Crossfading: Concerns for COVID-19 Disease Severity
- 1. Department of Biological and Biomedical Sciences, North Carolina Central University
- 2. Department of Epidemiology, UNC Chapel Hill
- 3. Business School, North Carolina Central University
Abstract
Risk factors for severe COVID-19 pathology are currently being investigated worldwide. Using a mouse model, we identify heavy alcohol and cannabinoid consumption as risk factors for increased pulmonary pathology in the setting of exposure to a microbial pulmonary pathogen (K. pneumoniae). We present observational evidence that pneumonia patients admitted to North Carolina hospitals have longer lengths of stay when they endorse alcohol use or have conditions considered alcohol-attributable. We are concerned that the observed increase in alcohol and legal cannabinoid sales during lockdown and quarantine may contribute to increased pulmonary pathology among patients who become infected with in COVID-19.
Key Points: Heavy alcohol and cannabinoid consumption prior to pneumonia infection resulted in increased severity of disease in mice . Analysis of hospital discharge data shows increased length of stay among alcohol-consuming pneumonia patients. Increased alcohol and cannabis sales during the early months of 2020 suggest that consumption of both substances has increased significantly during the pandemic, potentially representing increased risk of severe COVID-19 mediated by lung inflammation
Citation
Sivaraman V, Richey M, Nasir ABM (2021) Alcohol, Cannabis and Crossfading: Concerns for COVID-19 Disease Severity. J Subst Abuse Alcohol 8(1): 1088.
PERSPECTIVE
Historically, heavy alcohol usage has been associated with multiple pathologies, ranging from gastro-intestinal and renal toxicity to neurological dysfunction (1-3). The evidence supporting a relationship between alcohol abuse and more severe pulmonary disease is well documented in the medical literature (4). For example, incidence of both community-acquired and hospital-acquired pneumonias (CAP/HAP) have been observed to be more common among those who abuse alcohol (21, 22). Alcohol abuse is also associated with pneumonia outcomes, including longer times to recovery, more severe bacteremia, and increased mortality (5). Further evidence suggests that the severity of ARDS and pneumonia is greatly increased in those who have a history of alcohol and polysubstance abuse (4, 6).
Cannabis use has also been associated with pulmonary dysfunction, such as wheezing, shortness of breath and cough (7- 9). However, restrictions on the study of controlled substances and challenges in study design have resulted in conflicting evidence of a causative relationship between cannabis use and frank pulmonary disease (10-13). Similar to pathologies associated with alcohol abuse, cannabis abuse is associated with various pulmonary pathologies such as bronchial damage, basal cell hyperplasia and increased frequency and severity of asthma exacerbations (12,14). The practice of consuming alcohol and cannabis simultaneously, colloquially known as “crossfading,” is known to be common among cannabis users, and has only recently been a target of investigation (15). Recent study into the effect of simultaneous cannabis and alcohol consumption suggests that cannabis modulates the pulmonary inflammatory effects of alcohol via toll-like receptors (TLRs) that sense pathogens and initiate inflammatory responses (16). Many states have legalized medicinal and recreational cannabis usage, highlighting the need to understand the effects of simultaneous alcohol and cannabis usage.
The novel coronavirus SARS-CoV-2 has presented as a highly communicable respiratory virus and the causative agent for the lethal pulmonary disease known as COVID-19. SARS-CoV-2 can easily be transmitted between people in close proximity through respiratory droplets or microscopic aerosolized particle. As of March 1, 2021, over 2.5 million fatalities associated with COVID-19 have occurred throughout the world, with the majority occurring in the United States, India, Brazil, Russia and Western Europe (United Kingdom, Italy, Spain and France) (https:// coronavirus.jhu.edu/map.html). While the pathology initially was thought to be limited to aged adults with various pre-existing health risk factors, otherwise healthy individuals have died from the disease and determinants of susceptibility are still being investigated. Environmental and social factors may contribute to the increase in pathology observed during COVID-19 disease. We are concerned that as SARS CoV-2 pandemic continues to spread, excessive alcohol and cannabis consumption, possibly associated with the stress of isolation, quarantine and social distancing, may increase the risk )of severe disease among those infected with COVID-19.
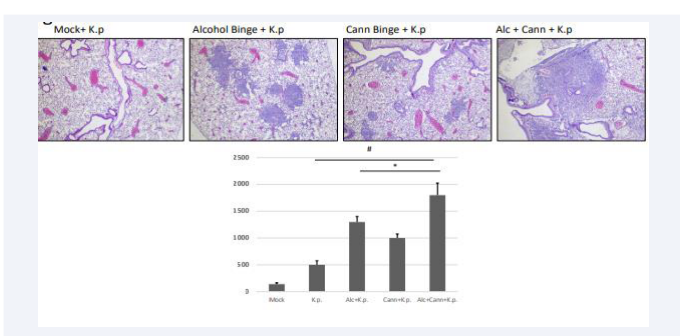
We have found evidence that excessive alcohol consumption exacerbates pulmonary inflammation in a mouse model, and is associated with increased disease severity (increased pneumonia incidence) and mortality during a microbial infection. We exposed 10 adolescent mice (C57-Bl6) to bingelevel quantities of ethanol (5 mg/kg, day on/day off over 10 days), allowed a two-week washout period, then challenged them with a sub-lethal intranasal dose of K. pneumoniae20 (a common nosocomial pulmonary infectious microbe). Our results demonstrate more severe pathology (increased neutrophilic influx within alveolar space demonstrated by H&E staining, Fig 1A)
Figure 1a : Mouse Lung Histopathology and Inflammatory Protein Secretion.
in the lungs of alcohol exposed animals, compared to control mice exposed only to K. pneumoniae. Experiments performed to evaluate the mechanism behind alcohol-mediated pulmonary exacerbation have implicated the cannabinoid receptordependent signaling cascade (unpublished data, in submission). In subsequent experiments, we exposed adolescent animals to either alcohol, an endogenous cannabinoid (WIN 55,212), or both, and then inoculated with K. pneumoniae intranasally (as previously described), and these experiments yielded similar results to alcohol exposure (Figure 1A). Cannabinoid-exposed mice demonstrated more severe disease compared to mock infected animals, as evidenced by increased inflammatory cytokines within pulmonary tissue, clinical signs of disease (increased respiration rate, hunching and ruffling) and increased pathology (neutrophilic influx, vascular edema). The mice that received both cannabinoids and alcohol had the most extensive neutrophilic influx, suggesting amplified effects of the two substances. Inflammatory cytokine protein IL-6 a common measure of inflammation, was assessed from collected bronchioalveolar lavage fluid from euthanized mice. Both singly-exposed groups of mice (alcohol, cannabis) displayed significantly higher levels of IL-6 compared to mice only exposed to K.p, while the doubly-exposed group (alcohol and cannabis) displayed the highest levels of IL-6. This is consistent with our hypothesis that cannabinoid signaling cascades contribute to severe pulmonary disease. These results suggest a role both for alcohol alone and the combination of alcohol and cannabis in increased morbidity and mortality when challenged with a microbial infection. We propose that while these preliminary results are currently applicable only to mice, the possibility that similar mechanisms are present in humans should be investigated.
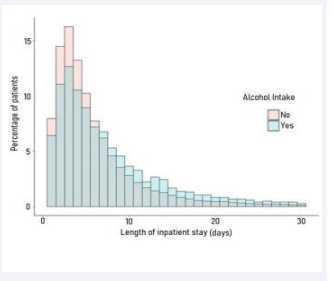
To evaluate whether alcohol exposure was also associated with more severe disease in humans, a retrospective cohort study using data from North Carolina hospitals was performed. We leveraged the large North Carolina Hospital Discharge Database, which contains records for all non-federal hospital discharges in the state. During 2016-2017, 14,685 patients age 18-75 were admitted with a primary diagnosis of pneumonia to North Carolina hospitals. We stratified these patients into two groups; one which endorsed alcohol use or had a history of disease considered 100% attributable to alcohol (N=738), and a second group with no history of alcohol related disease or endorsement of alcohol use (N=14,127). Multivariable linear and poisson regression models were employed to estimate the differences in length of stay between the two groups, adjusted for relevant confounders (age, sex, race). Patients in the alcohol group stayed a model-estimated 1.1 days longer (95% confidence interval: 1.0, 1.2 days p<.001) in the hospital compared to the non-alcohol group (Figure 1B).
Figure 1b: NC Hospital Discharge Data
This small but significant increase in length of stay is similar in magnitude with other studies of alcohol as a risk factor for contracting pneumonia (21,22) inpatient length of stay (5,23-25) necessity of mechanical ventilation, and incidence of acute respiratory distress syndrome (ARDS) (26). While our results are consistent with other studies in the literature, cautious interpretation of the validity of our estimate is warranted due to limitations in controlling confounding in hospital claims data, and the possibility of inconsistent or deliberate non-disclosure of alcohol use by hospital patients.
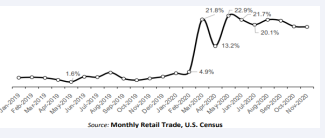
By March and April, much of the US enacted “Shelter-atHome” policies and encouraged social distancing to curtail the spread of the SARS-CoV2 virus. During this time of shelter at home and social distancing, businesses such as ABC stores and Cannabis dispensaries, (in 9 of 11 states where legal) were deemed “necessary” and remained open for business. US alcoholic beverage sales abruptly increased by 21.8% in March 2020 over the same month in 2019 (Figure 1C).
Figure 1c : The Monthly Retail Trade statistics data.
As the panic subsided, the sales dropped slightly by 13.2% in April, followed by another increase by 22.9% in May, and remained significantly higher through November of 2020. These results were robust to controlling for time trend, which suggests that the sudden increase in sales following the announcement of lockdown equates to panic buying.
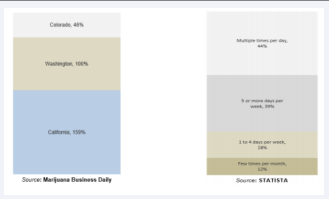
Figure 1D illustrates the dramatic increase in retail sales of marijuana due to COVID-19 outbreak in four U.S. states during March 2020. According to the report by Marijuana Business Daily, the sales of recreational marijuana in California increased by 159% compared to the sales reported on the same day in 2019, while sales in Washington and Colorado increased by 100% and 46%, respectively. The COVID-19 outbreak seems to be a contributing factor to the spike in cannabis sales as average year-over-year sales on Mondays between January 6 and March 9 were up by 71%, 10%, and 15%, respectively, in California, Colorado, and Washington (Figure 1D).
Figure 1d: Data obtained from Colorado, Washington, and California.
As observed from self-reported data across the US, the rate cannabis use during COVID-19 outbreak in the U.S. in 2020 increased (Fig 1D). This survey was conducted by Brightfield Group during the period spanning March 16 -19, 2020, finding that the heaviest cannabis users reported intentions to increase their usage during the coronavirus outbreak, and that the proportion of respondent who used cannabis multiple times per day increase by 32 percentage points. These data do not consider the likely dramatic increase in sales of both illicit and legal cannabis.
In conclusion, our data suggests that alcohol and cannabis use may contribute to synergistic priming of inflammation within the lung in the setting of pulmonary infection. In vivo mouse models, the clinical literature and population-based data supports the hypothesis that consumers of alcohol are both more prone to contracting pneumonia and to experience protracted illness. The observed increase of alcohol and cannabis sales may be a coping strategy against the isolation and stress associated with the pandemic. It is of great concern that as our population attempts to minimize their likelihood of contracting COVID-19 by isolating and distancing, they may be consuming compounds that may increase their vulnerability to severe disease.
REFERENCES
1. Crews FT, Vetreno RP, Broadwater MAand Robinson DL. Adolescent Alcohol Exposure Persistently Impacts Adult Neurobiology and Behavior. Pharmacol Rev. 20165; 68: 1074-1109.
2. Marks V. Clinical pathology of alcohol. J Clin Pathol. 1983; 36: 365-378.
3. Montoya ID. The pathology of alcohol use and abuse. Clin Lab Sci. 2013; 26: 15-22.
4. Boe DM, Vandivier RW, Burnham E L and Moss M. Alcohol abuse and pulmonary disease. J Leukoc Biol. 2009; 86: 1097-1104.
5. Saitz R, Ghali WA and Moskowitz, M. A. The impact of alcohol-related diagnoses on pneumonia outcomes. Arch Intern Med. 1997; 157: 1446-1452.
6. Wilson KC and Saukkonen JJ. Acute respiratory failure from abused substances. J ntensive Care Med. 2004; 19: 183-193.
7. Bloom JW, Kaltenborn WT, Paoletti P, Camilli A and Lebowitz MD. Respiratory effects of non-tobacco cigarettes. Br Med J (Clin Res Ed). 1987; 295, 1516-1518.
8. Tashkin DP, Simmons MS, Coulson AH, Clark VA and Gong H Jr.Sivaraman V, et al. (2021) J Subst Abuse Alcohol 8(1): 1088 (2021) 4/4 Respiratory effects of cocaine “freebasing” among habitual users of marijuana with or without tobacco. Chest. 1987; 92: 638-644.
9. Taylor DR, Poulton R , Moffitt TE, Ramankutty P and Sears MR. The respiratory effects of cannabis dependence in young adults. Addiction. 2000; 95: 1669-1677.
10.Reid PT, Macleod, J. & Robertson, J. R. Cannabis and the lung. J R Coll Physicians Edinb. 2010; 40: 328-323; quiz 333-324.
11.Ribeiro LI and Ind PW. Effect of cannabis smoking on lung function and respiratory symptoms: a structured literature review. NPJ Prim Care Respir Med. 2016; 26: 16071.
12.Lee MH and Hancox RJ. Effects of smoking cannabis on lung function.Expert Rev Respir Med. 2011; 5: 537-546; quiz 547.
13.Tashkin DP. Vaping Cannabis and Chronic Obstructive Pulmonary Disease. Ann Am Thorac Soc. 2018; 15: 1137-1138.
14.Goyal H, Awad HH and Ghali JK. Role of cannabis in cardiovascular disorders. J Thorac Dis. 2017; 9: 2079-2092.
15.White J, Walton D and Walker N. Exploring comorbid use of marijuana, tobacco, and alcohol among 14 to 15-year-olds: findings from a national survey on adolescent substance use. BMC Public Health. 2015; 15: 233.
16.Bailey KL. Joseph Sissona H, Debra Rombergerab J, Ellen Burnhamdet L, Art Heires J et al. Alcohol and cannabis use alter pulmonary innate immunity. Alcohol. 2019; 80: 131-138.
17.Subbaraman MS. Substitution and Complementarity of Alcohol and Cannabis: A Review of the Literature. Subst Use Misuse. 2016; 51: 1399-1414.
18.Clark TT, Doyle O and Clincy A. Age of first cigarette, alcohol, and marijuana use among U.S. biracial/ethnic youth: a population-based study. Addict Behav. 2013; 38: 2450- 2454.
19.Winters KC and Lee CY. Likelihood of developing an alcohol and cannabis use disorder during youth: association with recent use and age. Drug Alcohol Depend. 2008; 92: 239-247.
20.Harris B, McAlister A, Willoughby T and Sivaraman V. AlcoholDependent Pulmonary Inflammation: A Role for HMGB-1. Alcohol. 2018. 80: 45-52.
21.de Roux A. Manuela C, Maria AM, Elisa G, Santiago E et al. Impact of alcohol abuse in the etiology and severity of communityacquired pneumonia. Chest. 2006; 129, 1219-1225.
22.Fernandez-Sola J, Junqué A, Estruch R, Monforte R, Torres A et al. Highalcohol intake as a risk and prognostic factor for community-acquired pneumonia. Arch Intern Med. 1995; 155: 1649-1654.
23.Garau J, Baquero F, Pérez-Trallero E, Pérez J-L, Martín-Sánchez AM et al. Factors impacting on length of stay and mortality of communityacquired pneumonia. Clin Microbiol Infect. 2008; 14: 322-329.
24.Gili-Miner M, López-Méndez J , Béjar-Prado L , Ramírez-Ramírez G , Vilches-Arenas A et al. Alcohol Use Disorders and CommunityAcquired Pneumococcal Pneumonia: Associated Mortality, Prolonged Hospital Stay and Increased Hospital Spending. Arch Bronconeumol. 2015; 51: 564-570.
25.Gupta NM, Lindenauer PK, Yu PC , Imrey PB , Haessler S et al. Association Between Alcohol Use Disorders and Outcomes of Patients Hospitalized With Community-Acquired Pneumonia. JAMA Netw Open. 2019; 2: e195172.
26.de Wit M, Best AM, Gennings C, Burnham EL and Moss M. Alcohol use disorders increase the risk for mechanical ventilation in medical patients. Alcohol Clin Exp Res. 2007; 31: 1224-1230.