Oxytocin Co-Administered With Low-Dose Naltrexone Decreased Excessive and
- 1. Laboratory of the Biology of Addictive Diseases, The Rockefeller University, NY
ABSTRACT
Oxytocin is a neuropeptide that has potential for the development as an anti-alcoholism treatment. Clinical studies have recently found that intranasal oxytocin treatment reduces alcohol withdrawal symptoms or craving in alcohol-dependent patients with high anxiety. In rodents, activation of oxytocin receptor by systemic or central administration of oxytocin decreases alcohol reward, consumption and cue-induced alcohol seeking behaviors. The neurobiological interaction between oxytocin and mu-opioid receptors (MOR) has been well established: MOR agonists or endogenous beta-endorphin inhibit oxytocin release and neuronal activity. Here we explored whether oxytocin under MOR antagonism by naltrexone can enhance the reduction of alcohol intake by oxytocin alone after 3-week excessive alcohol drinking in an intermittent access alcohol (IAA) mouse model or after 1-week abstinence in mouse alcohol deprivation effect (ADE) model. For a genetic control for naltrexone effect, neuronal proopiomelanocortin enhancer (nPE) knockout mice with brain-specific beta-endorphin deficiency were further studied with oxytocin. We found that administration of oxytocin at 0.1 mg/kg [but not 0.01 or 0.03 mg/kg] decreased alcohol intake and preference. When oxytocin co- administered with naltrexone, oxytocin at 0.01-0.03 mg/kg with low doses of naltrexone (0.5 or 1 mg/kg) reduced alcohol drinking more profoundly than the sub-effective doses of oxytocin alone. Alcohol “relapse” in the ADE was prevented by either oxytocin alone or co-administration of oxytocin with naltrexone. The oxytocin effect was confirmed in nPE-/- mice, suggesting independent mechanisms by which oxytocin and naltrexone reduced alcohol drinking. Our study suggests that oxytocin in combination with low-dose naltrexone offers a novel strategy in alcoholism treatment.
KEYWORDS
• Alcohol deprivation effect
• Excessive alcohol drinking
• Combined therapy
• Low-dose naltrexone
• Oxytocin
Cite this article
Zhou Y, Baehr A, Zhou DC, Kreek MJ (2022) Oxytocin Co-Administered With Low-Dose Naltrexone Decreased Excessive and “Relapse” Alcohol Drinking In Mice. J Subst Abuse Alcohol 9(1): 1096.
INTRODUCTION
Central neuropeptide oxytocin and its G protein-coupled receptor oxytocin receptor (OXTR) are highly expressed in several brain regions, including the hypothalamus, mesolimbic and extended amygdala structures (1-2). Alterations of central oxytocin/OXTR system have been found both in rodents and humans after chronic alcohol exposure or withdrawal: (a) a persistent decrease of oxytocin is found in the hypothalamus of rodents (3-5) and humans (6), with increased plasma levels in humans during abstinence (7); and (b) OXTR mRNA and binding levels are upregulated in several forebrain regions of the alcoholdependent rats and in similar brain post-mortem regions of human alcoholics (prefrontal cortex, striatum, amygdala and hippocampus) (4). Therefore, the brain oxytocin “deficiency” with enhanced OXTR activity could be involved in the homeostatic adaptations of the neuronal circuits after chronic alcohol exposure and in the negative affective state during withdrawal (8-9). Consistently, pharmacological studies have demonstrated that systemic or central administration of oxytocin decreases alcohol reward (10) and alcohol consumption in mice and rats (8, 11-14), and blocks cue-induced alcohol seeking in the alcohol “dependent” rats (4, 15-16).
In the first clinical study, intranasal oxytocin treatment is found to reduce alcohol withdrawal symptoms in alcoholdependent patients (17). A separate clinical study demonstrated that intranasal oxytocin decreases cue-induced alcohol cravings in the patients with high anxiety (18). A human functional magnetic resonance imaging study found that intranasal oxytocin reduces neural reactivity to alcohol-related cues in human heavy social drinkers (4). Though these results indicate that oxytocin has potential utility in treating alcohol abuse or dependence, recent clinical data have found that oxytocin treatment either has modest therapeutic value or negative outcome (19-22).
By targeting multiple neurotransmitter systems involved in diverse components of alcohol addiction, combination medications may have increased efficacy over the singlemedication approach. In human alcoholics, MOR antagonist naltrexone decreases excessive alcohol consumption, craving and relapse episodes (23). As both naltrexone and oxytocin have been used in the clinical treatment of alcoholism or other diseases, the two compounds are two ideal candidates for investigating the potential benefit of combined treatments. In pre-clinical studies, the combinations of low-dose naltrexone with acamprosate or V1b antagonists have also demonstrated greater effects than either drug alone, with less adverse effects (24-26). Here, we hypothesized that oxytocin combined with low doses of naltrexone could synergistically reduce excessive alcohol drinking in mice, and our results may provide new information about the medical potential of oxytocin in the treatment of alcoholism. Therefore, the main subject of the present study is to test whether excessive alcohol drinking could be effectively reduced by the proper combinations of these two drugs, given that the two molecules have different mechanisms of actions (OXTR agonism for oxytocin and MOR antagonism for naltrexone).
For this purpose, the first objective was to evaluate the pharmacological effect of oxytocin alone in mice after 3-week intermittent access alcohol (IAA) drinking and after 1-week abstinence [the alcohol deprivation effect (ADE)], which mimic excessive, escalation and “relapse” alcohol intake (26) (5). To confirm that the oxytocin effect was OXTR mediated, we further tested whether selective OXTR antagonist L-368,889 could block the oxytocin effect (13). After the sub-effective doses of oxytocin that when given alone had no effect, were determined, we specifically tested several combinations of oxytocin and naltrexone using the sub-effective doses of each drug. The sub-effective doses of naltrexone (0.5 and 1 mg/kg) have been determined in our early studies using the same IAA paradigm in mice (26). To further explore a possible mechanism for the oxytocin synergistic effect with naltrexone, we tested whether oxytocin at a sub-effective dose would reduce alcohol intake in neuronal Pomc enhancers (nPE) knockout mice with central beta-endorphin deficiency as a genetic control for the naltrexone effect.
MATERIALS AND METHODS
Animals. Male or female adult C57BL/6J mice (8 weeks of age), obtained from The Jackson Laboratory (Bar Harbor, ME, USA), were placed on a 12-hour reverse light-dark cycle (lights off at 7:00 am) upon arrival, and acclimated for 2 weeks prior to testing. Mice were individually housed in ventilated cages fitted with steel lids and filter tops and given ad libitum access to food and water in a temperature-controlled room (21 ºC). Animal care and experimental procedures were conducted according to Guide for Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources Commission on Life Sciences 1996) and were approved by the Institutional Animal Care and Use Committee of the Rockefeller University.
Pomc neuronal enhancers 1 and 2 knockout mice. As reported in our earlier studies, we used male mice with targeted deletions of the two 5’ distal neuronal POMC enhancers (nPE-/-) combined with an insertion of a transcriptional blocking neo cassette within the enhancer locus (27). Pomc expression in the hypothalamic arcuate nucleus is decreased to < 5% in the mutant mice compared to controls, but there is no expression change in the pituitary, nucleus accumbens or brainstem medullary POMC cells (27) (5). The Pomc nPE-/- mice have been back-crossed onto the C57BL/6J strain for > 10 generations and bred in the Rockefeller University vivarium from a stock originally from Malcolm J Low at the University of Michigan.
Because the homozygous Pomc nPE-/- mice become obese and infertile, all breeding is performed using pairs of heterozygous Pomc nPE+/- mice. nPE-/- mice had more daily food intake (~5.2 g) than nPE+/+ mice (~3.3 g) at 8-9 weeks of age. At 10 weeks of age, nPE-/- (~40g) had more body weight than nPE+/+ (~29g).
Materials
Human synthetic oxytocin (CellSciences, Canton, MA), naltrexone hydrochloride (Sigma-Aldrich) and L-368,899 hydrochloride (Tocris Bioscience, Minneapolis, MN) were dissolved in physiological saline. Ethanol solutions were prepared from 190 proof absolute ethyl alcohol (PharmcoAAPER, Brookfield, CT, USA) and dissolved in tap water.
Procedures
Intermittent access (IAA) alcohol drinking. In C57BL/6J mice, this model has been widely used by many laboratories and described in detail in earlier reports (26). During alcohol drinking for 3 weeks in their home cages, mice had free access to food and water. Briefly, starting at 10:00 am (3 hours after lights off), both the alcohol (15% solution) sipper tube and water sipper tube were given on their home cages, and their position on left or right side of the cage was randomly placed to avoid any development of side preference. The tubes filled with fresh alcohol solution were placed for 24 hours before being substituted by the water tubes. Alcohol and water intake values were recorded at 0 hour and after 4, 8 and 24 hours of alcohol access in the drinking days, and these data were calculated as consumed alcohol intake (g ⁄ kg) and relative preference ratio for alcohol (alcohol intake ⁄ total fluid intake).
Oxytocin, oxytocin plus naltrexone or L-368,899 plus oxytocin administration(s) after IAA (Table S1A). On day 22, mice were randomly assigned as the vehicle- and drug- treated groups with similar alcohol intake in the baseline session. An experimenter, blinded to the experimental groups, injected the drug and vehicle. The mice in control groups received one vehicle injection or two; and the mice in test groups received one drug injection (oxytocin) or two drug injections (oxytocin followed by naltrexone). The oxytocin doses were based on an earlier publication (13): the mice in test groups received one oxytocin injection (0.01, 0.03 or 0.1 mg/kg, i.p.), and the mice in control groups received one vehicle injection (saline). The oxytocin plus naltrexone dose was based on the above experiments with oxytocin alone and our recent naltrexone study (26): the mice in test groups received the first i.p. injection of oxytocin (0.01 or 0.03 mg/kg) followed by the second i.p. injection of naltrexone (0.5 or 1 mg/kg in saline) 20 min later; and the mice in control groups received one vehicle followed by saline. Finally, the alcohol tube was given 10 min after the last drug or vehicle injection, and alcohol and water intakes were recorded after 4, 8 and 24 hours of alcohol access.
In female mice, we tested the effect of oxytocin at 0.1 mg/ kg after IAA, identical to the procedures in males. We observed comparable effects of oxytocin, suggesting that the estrous cycle and associated hormones might be unimportant in the response to oxytocin in females.
In male mice, we tested selective OXTR antagonist L-368,899 to block OXTR to confirm that the oxytocin effect was mediated via OXTR. Male mice were pretreated with L-368,889 (5 mg/kg) in saline (i.p.) 30 min before the drinking test, followed by oxytocin (0.1 mg/kg) or vehicle injection 10 min before the drinking test. The L-368,899 dose were based on an earlier publication (13).
In both nPE+/+ and nPE-/- male mice, alcohol was available 30 min after an injection of oxytocin or saline, and the sub-effective dose of oxytocin chosen (0.01 mg/kg) was based on the data in the above experiments. Mice were assigned to one of four groups: (1) nPE+/+ with vehicle; (2) nPE+/+ with oxytocin; (3) nPE-/- with vehicle; and (4) nPE-/- with oxytocin, and then alcohol and water intake values were recorded as described above.
Sucrose (caloric reinforcer) and saccharin (non-caloric reinforcer) drinking. The specific effect of oxytocin alone (0.1 mg/kg) or 0.03 mg/kg oxytocin combined with 1 mg/kg naltrexone on alcohol intake was further studied in sucrose or saccharin drinking. In these experiments, the IAA exposure was identical to those in the above experiment as described. After 3 weeks of IAA, the alcohol tube was substituted to sucrose for 3 sessions with stable intakes. The mice assigned to the vehicletreated or oxytocin-treated groups had similar sucrose intake 24 hours before the test day. On the test day, sucrose (4%) and water intake values were recorded after 4, 8 and 24 hours of sucrose access. In parallel separate experiments, saccharin drinking (0.1%) was tested after 3 weeks of IAA with an identical procedure.
The alcohol deprivation effect (ADE) after 1-week abstinence from IAA (Table S1B).
Like the above, mice accessed to alcohol for 24 hours on alternating days for 3 weeks. In the baseline session, 15% alcohol and water intakes were recorded at 4, 8 and 24 hours on day 21. The alcohol tubes were removed with only access to food and water. After 7 days of abstinence (day 30), new alcohol (30%) tubes were given to the mice 3 hours after the dark cycle and the alcohol and water intakes were recorded at 4, 8 and 24 hours in the ADE session (day 30). In the following two experiments, we tested the effect of oxytocin alone (0.1 mg/kg) or 0.03 mg/kg oxytocin combined with 1 mg/kg naltrexone in the ADE model. The mice assigned to the vehicle- or drug- treated groups had similar alcohol intake in the baseline session. Control groups: mice received one vehicle injection(s) before the ADE test; and Test groups: mice received oxytocin or oxytocin plus naltrexone before the ADE test. The doses chosen were based on the above IAA experiments.
Data analysis. We predicted that these studies require 6-8 males per group, based on the levels of differences seen previously (25) (26). In the IAA experiments, alcohol intake differences across the different groups were analyzed using two-way ANOVA for treatment (vehicle vs drug doses) and for times (4, 8 vs 24 h) with repeated measures, with testing our a priori hypothesis that there were effects of oxytocin, oxytocin with naltrexone or L-368,899 with oxytocin, based on the published findings (26) and our new hypothesis. In nPE mice, the group difference was analyzed using 2-way ANOVA for genotype (nPE+/+ vs. nPE-/-) and treatments (vehicle vs. oxytocin). In the ADE experiments, alcohol intake differences across the different groups were analyzed using two-way ANOVA for treatment (vehicle vs drug) and for session (baseline vs ADE), with testing our a priori hypothesis that there was an ADE based on the published findings (26). All the ANOVAs were followed by Newman-Keuls post-hoc tests. The accepted level of significance for all tests was p<0.05. All statistical analyses were performed using Statistica (version 5.5, StatSoft Inc, Tulsa, OK).
RESULTS
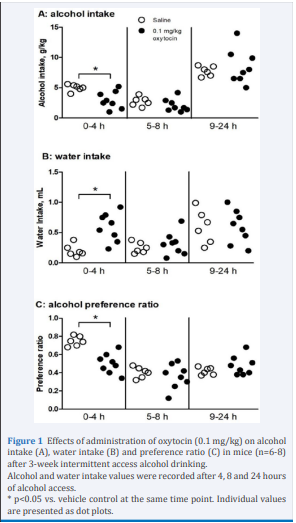
Oxytocin decreased alcohol intake and preference after IAA in a dose-related manner. At 0.01 or 0.03 mg/kg, oxytocin had no significant effect on alcohol intake, water intake or alcohol preference ratio (data not shown). At 0.1 mg/kg, however, there was a significant decrease in alcohol intake [2-way ANOVA, F(1,13)=5.9, p<0.05] at 4 hours [post-hoc test p<0.05] (Figure 1A), in comparison with the vehicle controls. This was coupled with a compensatory increase in water intake [F(1,13)=6.1, p<0.05] at 4 hours [p<0.05] (Figure 1B), resulting in nearly unchanged total fluid intake (Table S2A). At this dose, oxytocin also reduced preference ratio [F(1,13)=7.1, p<0.05] at 4 hours [p<0.05] (Figure 1C).
Figure 1 Effects of administration of oxytocin (0.1 mg/kg) on alcohol intake (A), water intake (B) and preference ratio (C) in mice (n=6-8) after 3-week intermittent access alcohol drinking. Alcohol and water intake values were recorded after 4, 8 and 24 hours of alcohol access. * p<0.05 vs. vehicle control at the same time point. Individual values are presented as dot plots.
A full-dose response of oxytocin administration (0, 0.01, 0.03 and 0.1 mg/kg) in alcohol intake and preference ratio at the 4-hour time point is presented in Figure 2.
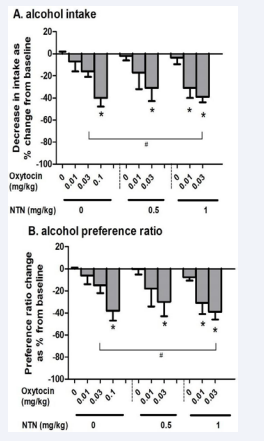
Figure 2: Dose responses of administration of oxytocin alone (0, 0.01, 0.03 or 0.1 mg/kg) and combined with naltrexone (0, 0.5 or 1 mg/kg) on reducing alcohol intake (A) and preference ratio (B) in mice (n=6-7) after 3-week intermittent access alcohol drinking. Data were collected at the 4-hour time point on the testing day and are expressed as a percentage of alcohol intake or preference ratio in vehicle-treated mice. * p<0.05 vs. control (oxytocin at 0 mg/kg), and # p<0.05 vs. 0.03 mg/kg oxytocin alone dose. Data are presented as mean + SEM.
In comparison to the control group, mean alcohol intake in the oxytocin-treated mice was reduced by 7% and 16% at 0.01 and 0.03 mg/kg oxytocin doses, respectively, with no significant effect. At 0.1 mg/kg, there were significant decreases on alcohol intake (~40%) [1-way ANOVA, F(9,62)=14, p<0.001; post-hoc test p<0.05 at 0.1 mg/kg] (Figure 2A, left) and on preference ratio [F(9,62)=12, p<0.001; p<0.05 at 0.1 mg/kg] (Figure 2B, left).
In this experiment, we tested oxytocin at 0.1 mg/kg after 3 weeks of IAA in female mice, in comparison with male mice (Figure 1). As there was no significant sex difference in the effect of oxytocin on alcohol consumption, the female data are presented separately in (Table 1). For intake, 2-way ANOVA with repeat measure revealed a significant effect of oxytocin [F(1,14)=5.0, p<0.05], with less intake than the vehicle control at 4 hours [p<0.05]. For preference ratio, there was a significant effect of oxytocin [2-way ANOVA, F(1,14)=5.5, p<0.05], with less preference than the vehicle-control at 4 hours [p<0.05].
Table 1. Effects of administration of oxytocin (0.1 mg/kg) on alcohol intake and preference ratio in female mice (n=8) after 3-week intermittent access alcohol drinking. Alcohol and water intake values were recorded after 4, 8 and 24 hours of alcohol access. * p<0.05 vs. vehicle control at the same time point. Data are presented as mean + SEM.
| time | Vehicle | 0.1 mg/kg oxytocin | |
| alcohol | 0-4h | 7.7 ± 1.1 | 4.5 ± 1.0 * |
| intake (g/kg) | 5-8h | 6.2 ± 0.7 | 5.8 ± 0.7 |
| 9-24h | 13 ± 1.0 | 14 ± 1.4 | |
| Preference | 0-4h | 0.84 ± 0.05 | 0.59 ± 0.07 * |
| ratio | 5-8h | 0.70 ± 0.04 | 0.66 ± 0.04 |
| 9-24h | 0.55 ± 0.06 | 0.56 ± 0.05 |
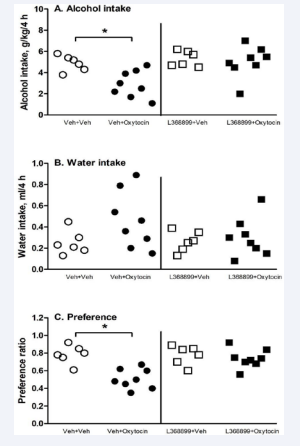
Pretreatment with L-368,899 blocked the oxytocin effect on alcohol drinking. For intake (Figure 3A), 2-way ANOVA showed significant effects of oxytocin [F(1,24)=7.1, p<0.05], L- 368,899 pretreatment [F(1,24)=6.5, p<0.05] and an interaction between L-368,899 and oxytocin [F(1,24)=6.6, p<0.05].
Figure 3: Pretreatment with selective oxytocin receptor antagonist L-368,899 (5 mg/kg) blocked the effect of oxytocin (0.1 mg/kg) on reducing alcohol intake (A) and preference ratio (C), but not water intake (B) in mice after 3-week intermittent access alcohol drinking (n=6-8). Data are presented after 4 hours of alcohol access. * p<0.05 vs. vehicle control with the same pretreatment. Individual values are presented as dot plots.
For preference ratio (Figure 3C), there was a significant effect of oxytocin [2-way ANOVA, F(1,24)=11, p<0.05]. At 0.1 mg/kg, oxytocin decreased alcohol intake and preference ratio [p<0.05 for both], and L-368,899 pretreatment at 5 mg/kg blunted the oxytocin effect..
Oxytocin combined with naltrexone dose-dependently decreased alcohol intake and preference ratio after IAA. As shown in (Figure 2), the effect of oxytocin (0, 0.01 or 0.03 mg/ kg) combined with naltrexone (0.5 or 1 mg/kg) on alcohol intake and preference was analyzed at the 4-hour time point. Oxytocin at a lowest dose (0.01 mg/kg) with naltrexone at 0.5 mg/kg did not reduce alcohol intake or preference. Combined with a higher dose of naltrexone at 1 mg/kg, however, 0.01 mg/kg oxytocin significantly reduced alcohol intake [p<0.05] (Figure 2A, right) and preference ratio [p<0.05] (Figure 2B, right). Oxytocin at a higher dose (0.03 mg/kg) with either 0.5 or 1 mg/kg naltrexone showed significant reductions in both intake and preference ratio [p<0.05]. The oxytocin with naltrexone (0.3 mg/kg plus 1 mg/kg) showed more reductions than 0.3 mg/kg oxytocin alone in both the intake and preference ratio [p<0.05] (Figure 2).
Table 2 presents alcohol and water intake values recorded at 4, 8 and 24 hours, following a combination dose [0.03 mg/kg oxytocin plus 1 mg/kg naltrexone]: significantly reduced alcohol intake [2-way ANOVA, F(1,10)=5.7, p<0.05] at 4 hours [post-hoc test p<0.05] and preference ratio [F(1,10)=5.8, p<0.05] after 4 hours [p<0.05]. This was associated with an increase in water intake at 4 hours [p<0.05]. The combination had no effect on total fluid intake (Table S2B).
Table 2. Effect of administration of oxytocin (0.03 mg/kg) combined with naltrexone (1 mg/kg) on alcohol intake and preference ratio after 3-week intermittent access alcohol drinking in mice (n=6). Data are presented after 4, 8 and 24 hours of alcohol access. *p<0.05 vs. vehicle control. Data are presented as mean ± SEM.
| time | Vehicle + Saline |
0.03 mg/kg oxytocin + 1 mg/kg naltrexone |
|
| alcohol | 0-4h | 6.1 ± 0.52 | 3.6 ± 0.35 * |
| intake (g/kg) | 5-8h | 4.1 ± 1.0 | 3.7 ± 0.70 |
| 9-24h | 8.7 ± 0.99 | 8.2 ± 1.0 | |
| Preference | 0-4h | 0.82 ± 0.07 | 0.51 ± 0.03 * |
| ratio | 5-8h | 0.53 ± 0.09 | 0.50 ± 0.06 |
| 9-24h | 0.54 ± 0.07 | 0.53 ± 0.07 |
No effect of oxytocin alone or combined with naltrexone on sucrose or saccharin drinking. As alcohol is a caloric reinforcer, the specificity of the oxytocin effect on alcohol was determined by testing its effect on sucrose (caloric reinforcer) and saccharin (non-caloric reinforcer) drinking after IAA. No effect of oxytocin at 0.1 mg/kg alone or oxytocin plus naltrexone (0.03 mg/kg plus1 mg/kg) (the most effective dose or combination for reducing alcohol) on 4% sucrose (Table S3A and S3B) or 0.1% saccharin (Table S3C and S3D) was found. Similarly, there was no effect of oxytocin alone or oxytocin plus naltrexone on sucrose or saccharin drinking in alcohol-naïve mice (data not shown).
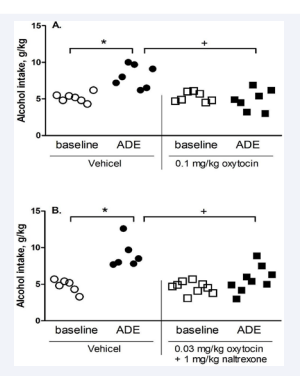
Oxytocin alone or combined with naltrexone decreased ADE. In a pilot, we tested the effect of oxytocin at 0.03 mg/kg alone and there was no significant effect on ADE (data not shown). At 0.1 mg/kg (Figure 4A), 2-way ANOVA revealed significant effects of oxytocin [F (1,24) = 4.9, p<0.05] and session and oxytocin interaction [F(1,24)= 4.8, p<0.05] at 4 hours: the oxytocintreated ones had less intake than the vehicle-treated ones in the ADE session [p<0.05]. To test our a priori hypothesis that an ADE occurred, the post-hoc test showed that in the vehicle- treated mice, the ADE was significant. There were no significant effects of ADE or oxytocin after 8 or 24 hours (data not shown).
Figure 4: Effect of administration of oxytocin (0.1 mg/kg) (A) (n=7) or oxytocin (0.03 mg/kg) plus naltrexone (1 mg/kg) (B) (n=6-9) on alcohol intake (g/kg) after 1-week abstinence (alcohol deprivation effect, ADE) from 3 weeks of intermittent access alcohol drinking in mice. Data are presented after 4 hours of alcohol access. * p<0.05 vs. vehicle control at baseline; and + p<0.05 vs. ADE. Individual values are presented as dot plots.
At 0.03 mg/kg oxytocin combined with 1 mg/kg naltrexone (Figure 4B), 2-way ANOVA revealed a significant effect of Session [F(1,26) = 4.7, p<0.05], and post hoc analysis showed that the control mice had more intake in the ADE session than the baseline [p<0.05]. To test our a priori hypothesis that the combination had an effect as oxytocin alone, post hoc analysis showed that the oxytocin plus naltrexone group had less intake than the vehicle control group in the ADE session [p<0.05]. Like the above experiment with oxytocin alone, there were no significant effects of ADE or oxytocin plus naltrexone after 8 or 24 hours (data not shown).
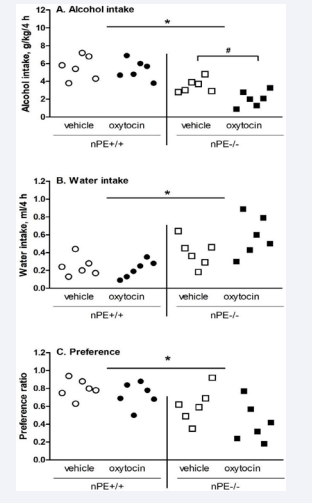
Oxytocin at a sub-effective dose (0.01 mg/kg) decreased alcohol intake after IAA in nPE mice. After 4 hours, 2-way ANOVA showed significant effects of genotype [F(1,20)=29, p<0.01] and oxytocin [F(1,20)=5.3, p<0.05] on alcohol intake (Figure 5A).
Figure 5: Genotype differences in the effects of oxytocin (0.01 mg/ kg) on alcohol intake (A), water intake (B) and preference ratio (C) after 3-week intermittent access drinking at 4 hours in male nPE mice (n=6). Genotype difference: * p<0.05 vs. nPE-/- mice; Oxytocin effect: # p<0.05 vs. vehicle control. Individual values are presented as dot plots.
Post hoc analysis showed that: (1) between genotypes, nPE+/+ had more intakes than nPE-/- [p<0.05]; and (2) oxytocin decreased intake in nPE-/- [p<0.05] only. For water intake, 2-way ANOVA showed a significant effect of genotype [F(1,20)= 5.7, p<0.05] ]: nPE+/+ had less water intake than nPE-/- (Figure 5B). For alcohol preference, 2-way ANOVA showed a significant effect of genotype [F (1, 20)=13, p<0.01]: nPE+/+ had more preference than nPE- /- (Figure 5C). Between 5-8 hours (Table S4A) and 9-24 hours (Table S4B), there were only significant genotype differences [F (1,20)=16, p<0.01] and [F(1,20)=12, p<0.05], respectively, confirming that nPE+/+ had more alcohol intake than nPE-/-, with no oxytocin effect.
DISCUSSION
The first objective in the present study was to investigate the potential of oxytocin alone in reducing excessive drinking in mice after 3-week IAA alcohol drinking. In the IAA model, an escalation of alcohol consumption after a period of excessive drinking has been considered to mimic a transition from alcohol abuse to dependence (26) (15). Using this IAA model, we found that administration of oxytocin alone significantly reduced alcohol intake and preference ratio in a dose-related manner (Figures 1 and 2). The effect of oxytocin on alcohol drinking was not due to its general inhibition of appetitive consumption (anhedonic effect), as oxytocin at the dose range tested did not change sucrose or saccharin intake (Table S3). As the effect of oxytocin on alcohol intake was blunted by selective OXTR antagonist L-368,889, our study further confirmed that the oxytocin effect was OXTR mediated (Figure 3). Extended with many reports of oxytocin effects on alcohol drinking behaviors, our new study demonstrate that oxytocin reduced excessive alcohol drinking through an OXTR-mediated mechanism (13) (16).
To examine whether oxytocin altered alcohol relapse-like drinking, the present study further investigated the potential of oxytocin in preventing ADE after 1-week abstinence from excessive alcohol drinking. ADE, as a rodent model, mimics the relapse episodes in human alcoholics, like relapse drinking and cravings (28). Specifically, we tested oxytocin at 0.03 or 0.1 mg/kg doses and found that oxytocin at 0.1 mg/kg significantly decreased ADE intake (Figure 4A). Hence, our findings that oxytocin decreased alcohol relapse-like consumption would extend the anti-relapse properties of oxytocin observed in other drugs related behaviors (2). As oxytocin/OXTR plays a counterbalance role with and vasopressin/V1b systems, the present study also extended early reports of V1b antagonists’ effect on alcohol “relapse” drinking behaviors (15) (25).
In our second objective, we provide initial evidence showing that the oxytocin plus naltrexone is more effective than either drug alone. Like the effect of oxytocin alone on reducing excessive drinking after IAA, the treatments with the oxytocin plus naltrexone efficiently decreased excessive drinking in mice. When compared the effects of oxytocin alone with the oxytocin plus naltrexone combination at low doses of each, we found that the combinations were more efficacious in reducing alcohol drinking than either drug alone. Our new results clearly demonstrate that oxytocin combining with low-dose naltrexone reduced excessive alcohol drinking in a mouse model, which would constitute an extension to the anti-alcoholism properties of oxytocin observed in rodent studies [see updated review by (29)]. The effect of this combination was alcohol-specific, as demonstrated by the lack of any effect on sucrose or saccharin consumption (Table S3). In the ADE model, the combination of oxytocin (0.03 mg/kg) and naltrexone (1 mg/kg) displayed a synergistic effect on preventing ADE (Figure 4B), as each compound at this low dose alone had no effect. In many cases, the single-receptor pharmacotherapies have been found to have modest therapeutic value over placebo, suggesting a need for better efficacy (26). Our studies in mouse IAA and ADE models have provided promising in vivo data demonstrating that the combination of clinically utilized oxytocin with naltrexone may be more efficacious in treating alcoholism than either oxytocin or naltrexone alone. By targeting multiple neurotransmitter receptors implicated in different components of alcohol addiction, combination medications are likely to have enhanced efficacy over the traditional single-receptor approach.
The mechanistic hypothesis that the oxytocin/OXTRmediated pathway has a different mechanism to drive excessive alcohol drinking from the MOR activation by endogenous beta- endorphin was further studied using nPE knockout mice lacking central beta-endorphin. Using the IAA model, we found that nPE-/- had lowered alcohol consumption, indicating a deficit of alcohol rewarding effect of alcohol in the nPE-/- as reported before (5, 26). Then we determined if the activation of OXTR by oxytocin could produce a decrease in alcohol intake further in nPE-/- as a genetic control for the naltrexone effect. As shown in Figure 5, nPE-/- showed a significant decrease in alcohol intake after oxytocin at a sub-effective dose (0.01 mg/ kg) in the nPE+/+ mice, indicating a sensitized effect of oxytocin with central POMC/beta-endorphin deficiency. The data also strongly suggest that the oxytocin effect in nPE-/- was through different mechanisms from that of naltrexone with MOR. As the effectiveness of the oxytocin plus naltrexone combination could involve multiple neuronal pathways (at least OXTR and MOR), the combination exhibited synergistic in reducing alcohol intake (Figure 2). Indeed, neurobiological studies have demonstrated that the multiple actions of alcohol in the brain include both the oxytocin/OXTR and endorphin/MOR systems which are profoundly altered by alcohol exposure in rodents (3) (4) (30) (5) (15) (29). Therefore, by aiming at both the OXTR and MOR involved in alcohol addiction, the combination of oxytocin and naltrexone is very likely to have enhanced efficacy over each single compound.
Finally, alcohol stimulates beta-endorphin release and POMC biosynthesis, and consequently, activation of MOR by betaendorphin produces rewarding effects, which play an important role in the reinforcing actions and motivational behaviors of alcohol drinking in rodents (31-33). Naltrexone, acting as a MOR antagonist, blocks MOR in the mesolimbic circuitries that decrease the alcohol rewarding effect (positive reinforcement). The activation of opioid receptors is also linked with an inhibition of oxytocin neuronal activity and release (34-36). The potential interaction of oxytocin/OXTR and endorphin/MOR systems is further supported by the co-localization of the OXTR and MOR expression in specific brain regions across rodents and humans (37). After chronic alcohol exposure, therefore, the oxytocin release, neuronal activity and expression could be suppressed by the increased activity of beta- endorphin and MOR as found before in both rodents and humans (3-6). In contrast, MOR antagonism by naltrexone may reverse the inhibition of oxytocin neurons and normalize the oxytocin deficiency, at least as we hypothesize here with the current data.
In conclusion, consistent with recent studies on alcohol consumption in rodents with different models, our new finding here has provided further promising in vivo data showing that oxytocin, in combination with naltrexone, suggests a novel strategy in alcoholism treatment, possibly with improved efficacy.
CONTRIBUTION TO THE FIELD STATEMENT
Alcoholism remains a huge public health problem with limited treatment alternatives. Recently, the oxytocin system is recognized as a potential new target for development of alcoholism treatments, as several earlier clinical studies demonstrated that intranasal administration of oxytocin decreases symptoms of alcohol withdrawal and craving in humans. However, the most recent clinical studies have found negative results, showing that the oxytocin treatment is not effective. Therefore, more effective treatment strategies with oxytocin will need to be developed in the preclinical studies. As mu-opioid receptors (MOR) antagonist naltrexone increases oxytocin release and neuronal activity, we explored whether oxytocin under MOR antagonism by naltrexone can enhance the reduction of alcohol intake by oxytocin alone. Using excessive alcohol drinking and alcohol deprivation effect mouse models, we found that oxytocin alone decreased alcohol intake and prevented “relapse” drinking. Of interest, when oxytocin co-administered with naltrexone, oxytocin at subeffective doses reduced alcohol drinking and prevented “relapse” more profoundly than oxytocin alone.
The oxytocin effect was confirmed in neuronal proopiomelanocortin enhancer knockout mice with brainspecific beta-endorphin deficiency, suggesting independent mechanisms by which oxytocin and naltrexone reduced alcohol drinking. Together, the present study suggests that oxytocin in combination with naltrexone offers a novel strategy in alcoholism treatment.
ACKNOWLEDGEMENT
This work was supported by NIH AA021970 (YZ), Robertson Therapeutic Discover Fund at the Rockefeller University (YZ). Specific thanks for Dr. Malcolm J Low at University of Michigan Medical School for the nPE mutant mice. Specifically with this article, we remember Dr. Mary Jeanne Kreek for her contributions in medical research on drug addiction diseases.
Contributors
YZ, DCZ designed the study, managed literature searches, undertook behavioral study, and conducted statistical analysis. YZ, AB, DCZ, MJK wrote the manuscript.
REFERENCES
5. Zhou Y, Liang Y, Low MJ, Kreek MJ. Nuclear transcriptional changes in hypothalamus of Pomc enhancer knockout mice after excessive alcohol drinking. Genes Brain Behav. 2019; 18: e12600.
10.Bahi A. The oxytocin receptor impairs ethanol reward in mice. Physiol Behav. 2015; 139: 321-327.