Adenoid Cystic Carcinoma of the Head and Neck Treated with Interstitial High Dose Rate Brachytherapy: A Case Series
- 1. Department of Radiation Oncology, University of California, USA
Citation
Ariani RT, Park SJ, Zaide L, Venkat PS (2025) Adenoid Cystic Carcinoma of the Head and Neck Treated with Interstitial High Dose Rate Brachytherapy: A Case Series. Ann Otolaryngol Rhinol 12(6): 1374.
INTRODUCTION
Adenoid cystic carcinoma (ACC) is a rare malignant neoplasm comprising less than 1% of all head and neck cancers [1]. It follows an indolent yet relentless clinical course characterized by aggressive locoregional recurrence and distant metastasis, owed to its predilection for subclinical spread and perineural invasion [2]. The standard of care for curative management of ACC is complete surgical resection followed by post-operative external beam radiotherapy (EBRT) [3]. However, infiltrative growth patterns often preclude complete surgical excision, underscoring the critical role of adjuvant radiotherapy in improving local disease control rates [4].
Despite the established efficacy of EBRT, durable local control remains difficult to achieve due to relative radioresistance and the proximity of critical structures that restrict safe dose escalation [5]. Interstitial brachytherapy (ISBT) provides a highly conformal technique capable of delivering high radiation doses directly to the tumor while sparing surrounding normal tissue [5]. Literature describing its use in head and neck ACC remains scarce and has primarily focused on low-dose-rate (LDR) Iridium-192 (Ir-192) seed-based ISBT. This series presents three patient cases with recurrent head and neck ACC treated with high dose-rate (HDR) ISBT, demonstrating its effectiveness in achieving precise focal dose escalation within anatomically constrained regions resulting in excellent local control with minimal associated toxicity.
CASE PRESENTATIONS
Case 1
A 65-year-old woman with multiply recurrent ACC of the right parotid gland was initially treated with right parotidectomy and neck dissection followed by adjuvant EBRT to a dose of 55 Gy. Approximately 11 years later, she developed a local recurrence near the right mandible that was managed with repeat resection followed by intraoperative (15 Gy) and postoperative (30 Gy) EBRT. Five years later, she developed another recurrence in the suprazygomatic region, which was resected with positive margins. No further adjuvant EBRT was recommended due to concern for cumulative dose to organs at risk. Over the following two years, two additional resections were performed for locally recurrent disease in the same area, both yielding positive margins. Roughly five years after the last surgery, she underwent resection for another local recurrence involving the right temporalis fascia.
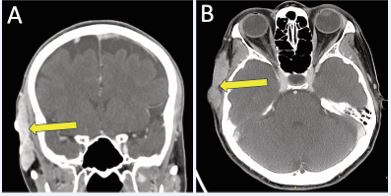
Three years later, serial imaging demonstrated increased thickening and enhancement along the right temporalis fascia. These findings continued to progress over the next six years with several enlarging subcutaneous nodules, which ultimately coalesced into a painful, 6 cm multilobular lesion along the right temporal scalp abutting the temporalis muscle and extending to the cutaneous surface without bony invasion, as demonstrated on contrast-enhanced head CT (Figure 1).
Figure 1 Contrast-enhanced CT head demonstrating right temporal scalp recurrence in A) coronal; and B) axial planes.
After multidisciplinary discussion, further surgical resection was deemed high-risk due to potential morbidity including facial nerve damage resulting in right forehead paralysis, trigeminal nerve damage resulting in paresthesia, aesthetic deformity, and impaired wound healing. As the patient retained normal forehead movement and sensation, the team aimed to preserve these functions. Given that additional EBRT was contraindicated given cumulative dose constraints, HDR-ISBT was selected as the most appropriate treatment approach.
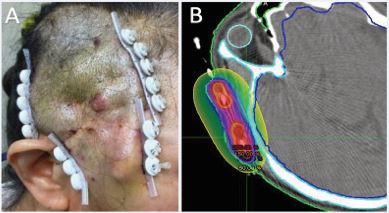
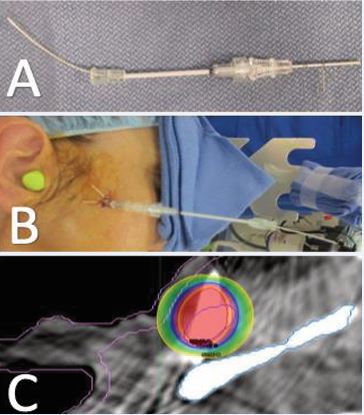
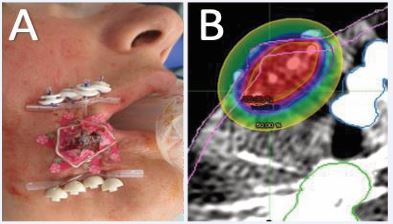
At the time of HDR-ISBT, examination revealed a large, irregular, firm lesion involving the right temple and extending superiorly onto the scalp, with areas adherent to the underlying skull. Intraoperative ultrasound was used to delineate tumor boundaries, assess depth, and guide catheter placement. The lesion exceeded 1 cm in thickness in several regions, with focal osseous abutment. Under monitored anesthesia care (MAC) and using subcutaneous and perilesional lidocaine and bupivicane, a complex, multi-planar interstitial implant encompassing the right temple and adjacent scalp was performed (Figure 2A).
Figure 2 Interstitial implant encompassing the right temporal lesion and adjacent scalp in (A) post op photo and (B) implant dosimetry with the 200% prescription dose in Red, the 150% in orange, the 100% in blue and the 50% in yellow, and the Clinical tumor volume (CTV) in purple.
The OncoSmart catheter system (OncoSmart, Elekta AB, Stockholm, Sweden) was used as a single-implant, multiple tube system. Eight flexible tubes were sequentially implanted in the target, beginning with the most difficult peripheral areas and progressing centrally to ensure even spatial distribution. 4F ProGuide afterloading treatment catheters were then inserted into the flexible implant tubes and connected to buttons secured at each catheter exit site for stability and identification during HDR source loading. The catheters were connected to the buttons only during treatment and removed between fractions to improve patient comfort. Intraoperative evaluation confirmed optimal catheter geometry, spacing, and coverage. The procedure was completed without complication and was well-tolerated by the patient.
Using an Elekta Flexitron (Flexitron, Elekta AB, Stockholm, Sweden) HDR afterloadingg system with a single Ir-192 source, a total dose of 40 Gy was delivered twice daily over one week in 4 Gy fractions (Figure 2B).Catheters were removed after completion of the treatment course without incident. The patient reported complete resolution of her tumor-associated pain and noted palpable tumor shrinkage within one-week post-treatment. Mild erythema developed two weeks post-treatment, followed by transient skin dryness, both of which resolved within one month.
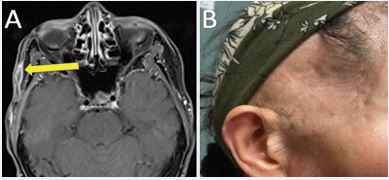
Contrast-enhanced face magnetic resonance imaging (MRI) obtained four months post-treatment demonstrated marked reduction in lesion size to 1.6 cm (Figure 3A).
Figure 3 (A) Contrast-enhanced face MRI four months post-treatment demonstrating significant treatment response. (B) Treated right temporal scalp at one-year post-treatment demonstrating excellent cosmetic outcome without clinical evidence of disease recurrence.
Routine surveillance MRI performed every six months thereafter showed stable findings with no evidence of local recurrence during two years of follow-up. No late toxicities, including nerve damage, fibrosis, or skin changes, were observed (Figure 3B).
Case 2
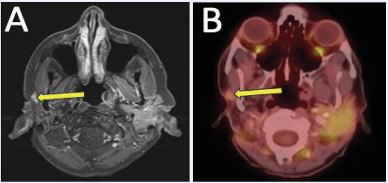
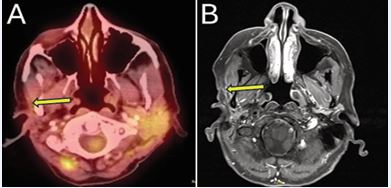
A 62-year-old woman with a history of ACC of the right parotid gland was initially treated with right deep lobe parotidectomy, which revealed pT3 disease with perineural invasion and positive margins. She subsequently received adjuvant EBRT (60 Gy) with concurrent carboplatin and paclitaxel. Approximately nine years after definitive treatment, surveillance contrast enhanced neck MRI demonstrated interval enlargement of enhancing soft tissue in the right parotid bed lateral to the right masseter muscle, previously interpreted as post surgical change, now measuring 1.2 cm (previously 0.7 cm) (Figure 4A).
Figure 4 (A) Contrast-enhanced neck MRI demonstrating enlarging soft tissue enhancement in the right parotid bed, suspicious for local recurrence. (B) PET/ CT confirmed increased FDG activity associated with recurrence.
Fine-needle aspiration performed confirmed recurrent ACC. Positron emission tomography/computed tomography (PET/CT) obtained for staging revealed mild FDG uptake at the site without evidence of metastatic disease (Figure 4B).
Following multidisciplinary review, the patient was deemed a poor surgical candidate due to the risk of facial nerve injury given the lesion’s location and the prior surgery and radiation to the area. As the patient retained full facial movement and prioritized preservation of function, surgical intervention was not recommended. Additional EBRT was contraindicated due to prior dose exposure. HDR-ISBT was therefore recommended to provide localized reirradiation while minimizing field overlap and skin dose, as the lesion was within a few millimeters of the skin surface.
The patient underwent three separate implant procedures under local anesthesia, each followed by same day delivery of a single treatment fraction. Implants were performed 3-7 days apart. Local anesthesia was achieved with subcutaneous and perilesional 2% lidocaine and 0.5% bupivacaine. Using palpation, ultrasound and CT guidance, a 3.8 cm coaxial introducer needle with an inner plastic afterloading catheter was inserted into the lesion, avoiding adjacent vascular structures. A CT contrast wire was then placed inside the inner plastic catheter. The needle was secured with a silk suture, and CT simulation confirmed optimal placement for dosimetric planning (Figure 5A, B).
Figure 5 Interstitial treatment device consisting of a CT contrast wire placed within the inner afterloading catheter within the coaxial introducer needle (A) prior to implantation and (B) implanted and secured in place. (C) Implant dosimetry with the GTV in purple, Skin in pink, mandible in gray, 200% prescription dose in red, 150% dose in orange, 100% dose in blue and 50% dose in yellow. As demonstrated, the majority of the CTV is receiving 200% dose. The 100% prescription dose is maintained off the skin, and the 50% dose is maintained off the mandible.
A total dose of 36 Gy in 3 fractions was delivered using the Elekta Flexitron afterloading system (Figure 5C). Implants were removed after completion of each fraction without complication, and the patient tolerated all procedures well.
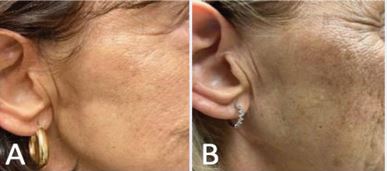
One week after completing treatment, the treated nodule was no longer palpable, and only mild self limited ecchymosis was observed. She developed subtle hyperpigmentation of the skin that remained stable following treatment (Figure 6).
Figure 6 Treated area with mild hyperpigmentation at (A) 3 months post treatment, which remained stable through (B) 4 years post-treatment.
PET/CT obtained three months post-treatment showed reduction of the lesion to 7 mm and complete resolution of FDG uptake, consistent with complete response (Figure 7A).
Figure 7 (A) PET/CT three-months post-treatment demonstrating resolution of FDG uptake. (B) Contrast-enhanced neck MRI obtained the following year redemonstrating no evidence of disease.
She continued routine contrast-enhanced MRI surveillance annually thereafter, with all imaging throughout four years of follow-up showing no evidence of residual or recurrent disease (Figure 7B). By two years after treatment, she reported only mild focal tightness at the treated site consistent with low-grade fibrosis, which has remained stable over time.
Case 3
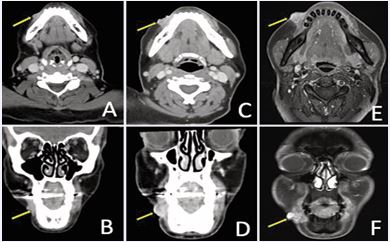
A 43-year-old woman with a history of cT2 ACC of the right lower lip was initially treated with EBRT (50 Gy) followed by an HDR-ISBT boost (20 Gy). She subsequently developed metastatic disease, managed with multiple lines of systemic therapy. Despite ongoing systemic therapy, approximately three years after completion of definitive radiotherapy, she presented with progressive enlargement and pain at the primary site. Contrast-enhanced face CT demonstrated nodular soft tissue thickening of the right lower lip measuring 0.8 cm (Figure 8A, B).
Figure 8 Recurrent right lower lip ACC demonstrated on contrast-enhanced face CT in (A) axial; and (B) coronal views. Repeat imaging one year later with interval growth, in (C) axial; and (D) coronal views. Contrast-enhanced face MRI four months later showing continued progression in (E) axial; and (F) coronal views.
Over the next year, imaging showed continued progression and necrosis with enlargement to 1.6 cm (Figure 8C, D). The lesion was closely adjacent to the right mental foramen without evidence of retrograde perineural spread along the inferior alveolar canal. Four months later, contrast enhanced face MRI demonstrated further enlargement up to 2.3 cm (Figure 8E, F).
Given prior irradiation, further EBRT was deemed unsafe due to cumulative dose constraints. However, she was considered a suitable candidate for repeat HDR-ISBT. At the time of HDR ISBT, examination revealed a 2 cm exophytic tumor involving the right commissure without oral mucosal involvement. Intraoperative ultrasound was used to identify the tumor, assess its depth, and guide catheter placement, ensuring appropriate spacing and alignment. Under monitored anesthesia care (MAC) and using subcutaneous and perilesional lidocaine and bupivicane, a complex, multi-planar interstitial implant encompassing the right commissure was performed. Using 17-gauge, 30-degree beveled hollow trocars, four afterloading catheters were sequentially inserted throughout the tumor volume (Figure 9A).
Figure 9 (A) Post-op photo of implant in place with CT wire to delineate target on CT simulation and (B) Plan Dosimetry with the 200% prescription dose in Red, the 150% in orange, the 100% in blue, the 50% in yellow, and the CTV in purple.
Catheters were positioned approximately 0.5 cm apart to encompass the lesion with a margin in all directions. Coded buttons were positioned at each catheter exit site for stability and identification during source loading. The implant was evaluated intraoperatively and confirmed to have proper geometry, spatial relationships, and target coverage. The procedure was completed without complication and was well-tolerated by the patient.
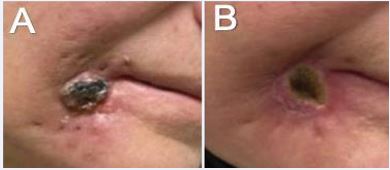
Using the Elekta Flexitron afterloading system, a total dose of 55 Gy was delivered twice daily over one week in 10 fractions (Figure 9B). Catheters were removed after completion of the treatment course without complication. At 2.5 months post-treatment, she reported complete resolution of pain, with the treated area noted to be flat, smaller, softer, and less indurated (Figure 10). Unfortunately, within 3 months of her last follow up, she had significant systemic disease progression and eventually succumbed to her disease, approximately 1 year after ISBT.
Figure 10 Necrotic right lower lip lesion A) pre-treatment; and B) 2.5 months post-treatment.
DISCUSSION
ACC of the head and neck remains difficult to manage due to its propensity for aggressive local recurrence [2]. Once recurrence develops after definitive surgery and EBRT, curative options are limited, as additional surgery or reirradiation often carry prohibitive morbidity [4,5]. In this series, HDR-ISBT was successfully used to treat three complex cases of locally recurrent or progressive ACC involving the scalp, parotid bed, and lower lip, each located within previously treated regions where further surgery or EBRT were contraindicated. All patients achieved durable local control, with complete or near-complete radiographic response and rapid symptom resolution.
Existing literature on ISBT for head and neck ACC remains limited and largely restricted to LDR seed-based techniques in the definitive rather than recurrent setting [5-10]. Published series have reported 3- and 5-year local control rates ranging from 68-94% [6-8] and 34-82% [6 10], respectively. In comparison, the two longest-followed patients in this series (Cases 1 and 2) had no evidence of local recurrence through two and four years of follow up, respectively. These results compare favorably with reported LDR-ISBT outcomes, particularly given the greater technical complexity and radioresistance inherent to the reirradiation setting. Similarly, a case report from Lee et al. described a case of recurrent ACC of the tongue base treated with HDR-ISBT after prior surgery and EBRT, achieving complete remission with no recurrence at two years of follow-up [11]. Additionally, treatment tolerance in our series of patients treated with HDR-ISBT was excellent, with no grade ≥2 toxicities experienced. This is in alignment with published experiences with LDR-ISBT in which most patients experienced only grade 1-2 acute and late toxicities [5-10]. The patient treated with HDR-ISBT for tongue base ACC described by Lee at al. developed only transient acute grade 2 mucositis which resolved within three weeks [11].
HDR-ISBT offers several advantages over LDR ISBT. Computer-controlled afterloading enables precise modulation of dwell positions and times to produce homogeneous and conformal dose distributions with steep dose gradients that limit exposure to critical normal structures such as the mandible, salivary glands, and cranial nerves [12-15]. Source repositioning and individualized dwell-time optimization facilitate safe and effective dose escalation of irregularly shaped targets [12]. Because HDR-ISBT is delivered over minutes rather than days, dosimetric uncertainties associated with anatomical shifts and patient movement are minimized relative to seed-based LDR-ISBT implants [16]. The ability to deliver fractionated treatment with HDR-ISBT permits sublethal damage repair in late-responding normal tissues, mitigating long-term toxicity [16]. Collectively, these attributes make HDR-ISBT particularly advantageous in the reirradiation setting, where precise geometric conformity and adaptive planning are essential.
Surface custom mold applications represent a noninvasive HDR brachytherapy approach that can effectively treat superficial lesions and are often employed in the treatment of head and neck cancers. The application of this technique for ACC was demonstrated in a case report from Reshko et al., in which a soft palate squamous cell and ACC collision tumor was successfully treated with surface mold brachytherapy [17]. However, for tumors with greater depth of invasion, this technique results in excessive skin dose while often inadequately covering the full tumor volume [18]. In such cases, as with our patients presented in cases 1 and 3, HDR-ISBT provides superior dosimetric control through placement of catheters at variable depths within the target, achieving comprehensive target volume coverage while maintaining acceptable skin dose constraints.
In summary, HDR-ISBT represents a safe and highly effective salvage option for complex, locally recurrent ACC of the head and neck. The outcomes achieved in this series parallel those reported with LDR-ISBT. HDR ISBT should be considered an important focal treatment strategy for patients with locally recurrent disease who are not candidates for additional surgery or EBRT. Future prospective studies are warranted to refine dose and fractionation parameters, assess long-term outcomes, and directly compare HDR and LDR techniques.
REFERENCES
- Fang Y, Peng Z, Wang Y, Gao K, Liu Y, Fan R, et al. Current opinions on diagnosis and treatment of adenoid cystic carcinoma. Oral Oncol. 2022; 130: 105945.
- Lorini L, Ardighieri L, Bozzola A, Romani C, Bignotti E, Buglione M, et al. Prognosis and management of recurrent and/or metastatic head and neck adenoid cystic carcinoma. Oral Oncol. 2021; 115: 105213.
- National Comprehensive Cancer Network. Head and Neck Cancers (Version 5.2025). 2025.
- Choi SH, Yang AJ, Yoon SO, Kim HR, Hong MH, Kim SH, et al. Role of postoperative radiotherapy in resected adenoid cystic carcinoma of the head and neck. Radiat Oncol. 2022; 17: 197.
- Huang MW, Zheng L, Liu SM, Shi Y, Zhang J, Yu GY, et al. 125I brachytherapy alone for recurrent or locally advanced adenoid cystic carcinoma of the oral and maxillofacial region. Strahlenther Onkol. 2013; 189: 502-507.
- Chen Y, Dai J, Jiang Y, Ji Z, Jiang P, Sun H, et al. Long-Term Outcomes of Personalized Stereotactic Ablative Brachytherapy for Recurrent Head and Neck Adenoid Cystic Carcinoma after Surgery or External Beam Radiotherapy: A 9-Year Study. J Pers Med. 2021; 11: 839.
- Dong S, Li W, Shi Y, Lv XM, Huang MW, Zhang JG. The efficacy of iodine-125 interstitial brachytherapy for the treatment of locally advanced adenoid cystic carcinoma of the base of tongue: a non- surgical approach. J Contemp Brachytherapy. 2021; 13: 395-401.
- Wu WJ, Shao X, Huang MW, Lv XM, Zhang XN, Zhang JG. Postoperative iodine-125 interstitial brachytherapy for the early stages of minor salivary gland carcinomas of the lip and buccal mucosa with positive or close margins. Head Neck. 2017; 39: 572-577.
- Gao Y, Zheng L, Zhang JG, Liu SM, Zhang JY, Dong S. Surgery combined with iodine-125 interstitial brachytherapy for treatment of parotid adenoid cystic carcinoma: A single-institution experience. Brachytherapy. 2021; 20: 383-392.
- Xu N, Zheng L, Wu WJ, Huang MW, Zhang J, Zhang JG. Definitive 125I Brachytherapy of Locally Advanced Adenoid Cystic Carcinoma Involving the Skull Base With Satisfying Efficacy and Safety. J Oral Maxillofac Surg. 2019; 77: 2143-2153.
- Lee SY, Kim JS, Kwon HC. High-dose rate brachytherapy for local recurrent adenoid cystic carcinoma of the tongue base following postoperative external beam radiotherapy. Mol Clin Oncol. 2016; 5: 500-502.
- Shwetha B, Ravikumar M, Katke A, Supe SS, Venkatagiri G, Ramanand N, et al. Dosimetric comparison of various optimization techniques for high dose rate brachytherapy of interstitial cervix implants. J Appl Clin Med Phys. 2010; 11: 3227.
- Banerjee R, Kamrava M. Brachytherapy in the treatment of cervical cancer: a review. Int J Womens Health. 2014; 6: 555-564.
- Wiegand S, Sesterhenn AM, Zimmermann AP, Strassmann G, Wilhelm T, Werner JA. Interstitial HDR brachytherapy for advanced recurrent squamous cell carcinoma of the head and neck. Anticancer Res. 2013; 33: 249-252.
- Rudzianskas V, Inciura A, Juozaityte E, Rudzianskiene M, Kubilius R, Vaitkus S, et al. Reirradiation of recurrent head and neck cancer using high-dose-rate brachytherapy. Acta Otorhinolaryngol Ital. 2012; 32: 297-303.
- Stewart AJ, Chargari C, Chyrek A, Eckert F, Guinot JL, Hellebust TP, et al. Radiobiology and modelling in Brachytherapy: A review inspired by the ESTRO Brachytherapy pre-meeting course. Clin Transl Radiat Oncol. 2024; 50: 100885.
- Reshko L, Khan Z, Sowards KT, Jordan A, Silverman C. Squamous Cell and Adenoid Cystic Carcinoma Collision Tumor of the Soft Palate Treated with Surface Mold Brachytherapy. Cureus. 2020; 12: e7297.
- Ouhib Z, Kasper M, Perez Calatayud J, Rodriguez S, Bhatnagar A, Pai S, Strasswimmer J. Aspects of dosimetry and clinical practice of skin brachytherapy: The American Brachytherapy Society working group report. Brachytherapy. 2015; 14: 840-858.