Alterations in the Oropharyngeal Microbiome after Adenoidectomy in Children with Adenoid Hypertrophy
- 1. Department of Otorhinolaryngology Head and Neck Surgery, The First Affiliated Hospital of Soochow University, China
- 2. Department of Otorhinolaryngology Head and Neck Surgery, Children’s Hospital of Soochow University, China
- 3. Jiangsu Institute of Hematology, The First Affiliated Hospital of Soochow University, China
- #. Both the authors contributed equally
Abstract
Objective: This study aimed to characterize the oropharyngeal microbial profiles of children with Adenoid Hypertrophy (AH) and assess changes following adenoidectomy.
Methods: Saliva samples were collected from 23 children who underwent adenoidectomy, both preoperatively and postoperatively. The samples were analyzed via 16S rRNA sequencing to determine the microbial composition. OTU abundance was normalized and used to assess microbial richness, diversity, and intergroup variability in microbiome structure.
Results: Preoperatively, the dominant bacterial genera included Streptococcus, Neisseria, Prevotella, Haemophilus, Actinomyces, and Gemella. Postoperatively, Streptococcus, Neisseria, and Prevotella remained prominent, with increased Leptotrichia and decreased Actinomyces. Significant differences in microbiome richness and diversity were demonstrated between preoperative and postoperative samples.
Conclusion: This study demonstrated the microbial diversity of the oropharyngeal microbiome in children with AH and evaluated the impact of adenoidectomy on this microbiome. Our findings contribute to understanding the relationship between alterations in the oropharyngeal microbiome and common clinical interventions for AH.
Keywords
• Adenoid hypertrophy
• Microbiota
• 16S RNA
• Oropharynx
• Adenoidectomy
Citation
Li J, Yuyang F, Depei Y, Shiyao Y, Xiaofei Q, et al. (2025) Alterationsin the Oropharyngeal Microbiome after Adenoidectomy in Children with Adenoid Hypertrophy. Ann Otolaryngol Rhinol 12(3): 1360.
INTRODUCTION
Adenoids, located in the nasopharynx and part of Waldeyer’s ring, serve as the initial defense mechanism against microorganisms entering the body via the mouth or nose [1,2]. These lymphoid tissues are particularly active in young children and are often implicated in various infectious and noninfectious upper airway conditions, such as otitis media, rhinosinusitis, and adenoiditis [3]. Adenoid Hypertrophy (AH), a common occurrence in children, may contribute to nasal obstruction, mouth breathing, snoring, and hearing impairment [4]. Surgical removal of adenoids, known as adenoidectomy, is frequently performed to alleviate recurrent ear infections, chronic adenoiditis, and Obstructive Sleep Apnea (OSA).Bacterial infection has been recognized as a significant factor in the development of AH, with common pathogens including Haemophilus influenza, Streptococcus pneumoniae, Streptococcus pyogenes, and Staphylococcus aureus [5]. In cases of chronic infections, anaerobic bacteria may also be present. Consequently, many children with AH harbor pathogenic bacteria in their nasopharynx, leading to the prescription of antibiotics during the perioperative period. A reduction in these pathogenic organisms is often observed following adenoidectomy [6]. Conventionally culture-independent molecular methods to identify and characterize microbiomes have been developed and used in studies of clinical microbiology [7]. Advancements in molecular methods have revolutionized the study of clinical microbiomes. Compared with traditional culture methods, Polymerase Chain Reaction (PCR) has improved the detection rate of pathogens, but it is limited to known bacteria. New sequencing technologies, such as 16S ribosomal RNA (16S rRNA) gene sequencing, offer a more comprehensive approach to microbial identification [8]. With the advancement of 16S rRNA sequencing, diverse microbial communities have been discovered on adenoids. The oropharynx and nasopharynx, adjacent anatomical structures in the upper throat, share a common environment that may facilitate interactions among their colonizing flora [9]. However, previous studies have relied primarily on culture-based methodologies, which identify only a fraction of known bacteria [10]. Furthermore, there is a paucity of research on the oropharyngeal microbiomes of children with AH. In this study, we aimed to characterize the oropharyngeal flora of AH patients and compare the microbial flora preoperatively and postoperatively for each individual via 16S rRNA sequencing. This approach provides insights into the microbial diversity and dynamics in the oropharynx of children with AH and the impact of adenoidectomy on the microbial flora.
MATERIALS AND METHODS
Patients
AH children participated in the study. All of them underwent adenoidectomy because of invalid conservative treatment at the Children’s Hospital of Soochow University, Suzhou, China. In our study, all the children were treated via nasopharyngeal X-ray and electronic epipharyngoscopy, which were used to evaluate the degree of adenoid hypertrophy. All the children were not treated with antibiotics for at least 4 weeks prior to sampling [11]. The exclusion criteria were the presence of any immunocompromised state, any heart condition necessitating endocarditis prophylaxis, severe infection, and any other systemic illnesses. The study was approved by the Medical Ethics Committee of Children’s Hospital of Soochow University, Suzhou, China. Written informed consent was obtained from all parents and legal guardians.
Sample collection
Samples from the oropharynx were analyzed for each subject. To obtain samples of the microbiome, individuals were asked to rinse their mouths with 20 mL of NS for a duration of 1 minute after refraining from eating, drinking, and oral hygiene practices for 2 hours. Samples of 10 mL of saliva naturally accumulated in the mouth were collected in sterile DNA- and RNA-free 15-mL centrifuge tubes and frozen immediately at − 80°C.
DNA extraction and sequencing analysis of 16S rRNA gene amplicons
Microbial genomic DNA was extracted from samples by combining freeze-grinding and sodium dodecyl sulfate for cell lysis and purified by agarose gel electrophoresis, followed by phenol?chloroform-pentanol extraction. The DNA was stored at -20°C for further use, diluted to a working concentration of 1 ng/μL, and sterile distilled water was used. The primers 515 F (5′-GTGCCAGCMGCCGCGG-3′) and 806 R (5′-GGACTACHVGGGTWTCTAAT-3′) targeting the V4 hypervariable regions of microbial 16S rRNA genes were selected. The library was sequenced on an Illumina MiSeq2*300 platform (TinyGene, Shanghai, China), and 500 bp paired-end reads were generated.
Bioinformatics and multivariate statistics
The samples were distinguished by introducing a barcode sequence into each sample’s sequence, which indicates the source information of the sample. Paired end reads from the original DNA fragments were merged via FLASH under the quality control of Trimmomatic software. UPARES software was used for sequence analyses. Sequences with ≥97% similarity were assigned to the same operational taxonomic units (OTUs). For each representative sequence, the SILVA database was searched via the Mothur method of annotating taxonomic information. OTU abundance information was normalized. Subsequent analyses of alpha diversity and beta diversity were performed on the normalized output data. Alpha diversity was used to assess the complexity of the microbial community for each sample via the Chao index, ACE index, Shannon index, Simpson index and Faith’s phylogenetic diversity (PD_whole_tree). The Chao index and ACE index reflect the species richness in a sample, whereas the Shannon index and Simpson index measure species diversity within a sample. All indices in our samples were calculated with QIIME (version 1.7.0) and displayed via R software (version 2.15.3; R Development Core Team, Vienna, Austria). The values were formally compared via the Tukey test. Beta diversity of weighted and unweighted UniFrac measurements was calculated via QIIME software (version 1.7.0). Principal Coordinate Analysis (PCoA) is a visualization technique utilized for examining the similarities or dissimilarities within data. Differences in the microbiota structure of the samples were assessed via Multiresponse Permutation (MRPP) procedures. Linear discriminant analysis effect size (LEfSe) was used to further assess the significant differential taxa between groups of samples. Significant differences in the microbiota from the phylum to the genus level were identified on the basis of the Linear Discriminant Analysis (LDA) score. A p value < 0.05 was the threshold for statistical significance.
RESULTS
Demographic data
The study included 23 consecutive children with AH who underwent adenoidectomy at the Children‘s Hospital of Soochow University, Soochow University, between July 2020 and August 2020. Samples were collected at the follow-up visit one month after the operation, and oropharyngeal cultures from 14 of the 23 patients included in the study were obtained both preoperatively and postoperatively. Nine patients were lost to follow-up. There were no statistically significant differences in sex or age.
Microbial composition
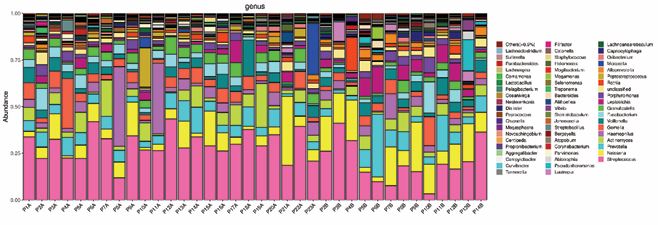
The most prevalent genera associated with adenoids (Streptococcus, Haemophilus and Neisseria) were also found in oropharyngeal microbiome samples. In addition, Prevotella, Actinomyces, and Gemella were also universally prevalent. The relative abundance of bacterial genera in individual samples is shown in Figure 1
Figure 1 The 16S ribosomal RNA (16S rRNA) gene-based bacterial community compositions of the samples from AH patients. The bacterial community sequence data are displayed at the genus level, with data for each taxon expressed as a proportion of sequence reads for a given sample. The relative abundance of bacterial genera is shown for individual samples.
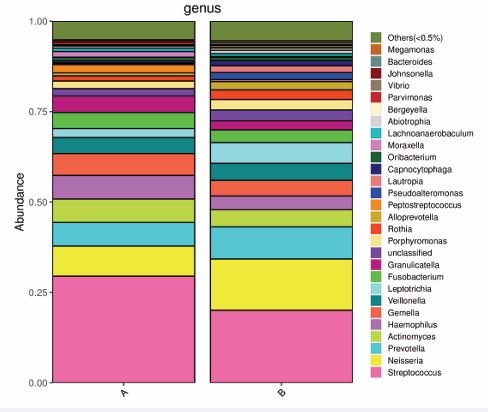
Figure 2 shows the mean relative abundances of the bacterial taxa associated with the top 30 genera at each site. In the preoperative groups (group A), the most abundant bacterial genus was Streptococcus (29.94%), followed by Neisseria (8.31%), Prevotella (6.57%), Haemophilus (6.56%), Actinomyces (6.45%) and Gemella (6.04%) (Figure 2). Streptococcus (20.07%), had the highest abundance in the postoperative samples of the AH children, followed by Neisseria (14.16%), Prevotella (8.91%), Leptotrichia (5.76%) and Actinomyces (4.72%) (Figure 2).
Figure 2 The 16S rRNA gene-based bacterial community compositions of the samples from the preoperative (Group A) and postoperative (Group B) groups at the genus level. The mean relative abundance of the bacterial taxa of the top 30 genera is displayed at each site.
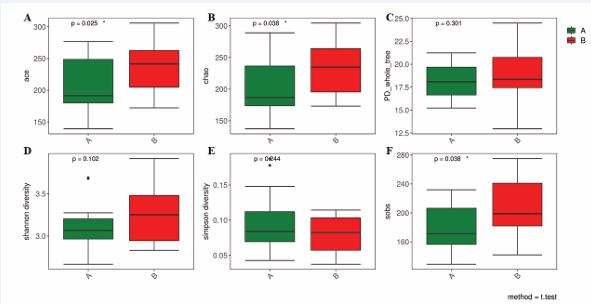
Alpha diversity of microbiomes
The species richness of oropharyngeal microbiomes in AH children was analyzed via the Chao1 index, the ACE index and the Sobs index. Diversity was evaluated by the Shannon index and Simpson index. There was a significant increase in the Chao index, ACE index and Sobs index (Figure 3A, Figure 3B and Figure 3F), between the preoperative (Group A), and postoperative (Group B), groups of AH patients. Species richness quantifies the diversity of species within a community, independent of the relative abundance of each species present. There were no significant differences in the Shannon index or Simpson index between the two groups (Figure 3D and Figure 3E). The alpha diversity indicated a similar level of diversity between preoperative and postoperative AH patients. PD_whole_tree (Figure 3C), also presented similar diversity. Notably, the richness in the postoperative groups was significantly greater than that in the preoperative groups. However, there was a nonsignificant increase in the diversity of the groups of AH patients following the surgical procedure.
Figure 3 Alpha diversity of the microbial community in each group analyzed by the Chao index (A), ACE index (B), PD_whole_tree (C), Shannon index (D), Simpson index (E) and Sobs index (F). Boxes represent the 25th to 75th percentiles; the bar indicates the media. (Green: preoperative group; Red: postoperative group).
Comparison of microbiomes between the preoperative group and the postoperative group
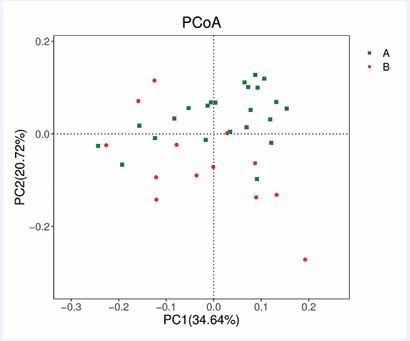
Differences in microbiota structure between the preoperative and postoperative groups of samples from AH children were estimated via several measures. Weighted UniFrac-based PCoA revealed distinct clusters of microbiota compositions (Figure 4).
Figure 4 Comparison of the β diversity of the microbial community in each group (A: preoperative; B: postoperative). Plots of weighted UniFrac-based PCoA in each group.
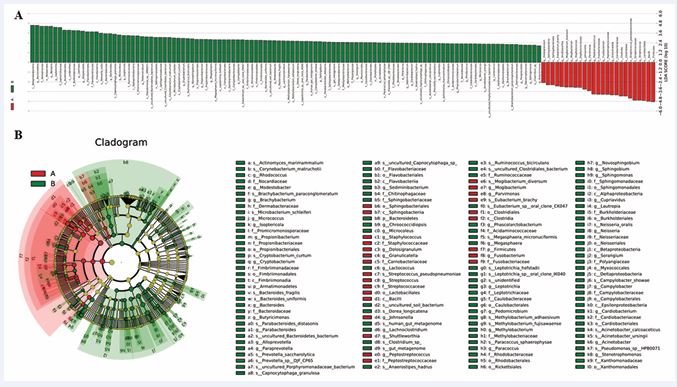
The results were further confirmed via MRPP, which revealed that the microbiome compositions of the postoperative samples were distinct from those of the preoperative samples in AH children (MRPP, A = 0.045, observed-delta = 0.254, expected-delta = 0.266, p = 0.002). LDA effect size (LEfSe) testing was used to further assess the significantly different taxa between the two groups of AH children. Different taxa were identified from the phylum level to the species level (Figure 5A,B).
Figure 5 Linear Discriminant Analysis (LDA) integrated with effect size (LEfSe) (red: preoperative; green: postoperative). (A) Differences in abundance between the two groups. (B) Cladogram indicating the phylogenetic distribution of microbiota correlated with the preoperative and postoperative groups.
At the genus level, the microbiota, including Fusobacterium, Peptostreptococcus, Granulicatella, and Streptococcus, were significantly overrepresented (all LDA scores (log10) > 3.9) in the samples from the preoperative group, whereas Alloprevotella, Lautropia, Neisseria, and Leptotrichia were the most abundant microbiota in the postoperative group (LDA scores (log10) > 3.8).
DISCUSSION
The adenoid, also termed the nasopharyngeal tonsil, occupies a central position within the nasopharynx, is situated posteriorly and superiorly to the choanae border and adjacent to lymphoid clusters encircling the nasopharyngeal orifices of the eustachian tube [12]. The rapid growth of adenoids during childhood is attributed to their crucial immunological functions [13]. The etiology of Adenoid Hypertrophy (AH) in pediatric patients remains incompletely understood but is likely intertwined with immune reactions, hormonal influences, and genetic predispositions [14]. Typically, a harmonious equilibrium exists between pathogens and the immune system; however, recurrent viral and bacterial infections can disrupt this balance. These disturbances can modify the entire oropharyngeal flora by allowing pathogenic bacteria to colonize, potentially contributing to chronic adenoiditis and predisposing children to other upper airway infections [15]. When conservative medical therapies fail, surgical intervention becomes necessary. In our study, we employed culture-independent methodologies such as PCR and 16S rRNA gene sequencing to characterize the bacterial communities in the oropharynx of children with AH. Our findings confirm the existence of diverse bacterial compositions in the oropharynx of these patients. Research has consistently identified Haemophilus influenza, Streptococcus pneumoniae, Streptococcus pyogenes, and Staphylococcus aureus as the most prevalent bacteria isolated from adenoid tissue [5]. Consistent with these findings, our study revealed that Gemella, Granulicatella, Streptococcus, and Veillonella were abundant in the normal oral cavity flora. Notably, certain Streptococcus species (e.g., S. mitis and S. salivarius), are associated with bacterial endocarditis [16]. Although our study did not provide species-level identification, Streptococcus, Neisseria, and Prevotella emerged as the most prevalent genera in AH children, reinforcing the notion that bacteria play a pivotal role in AH. Our findings align with those of previous studies that analyzed the adenoid microbiomes of AH patients [11,17,18]. By examining the oropharyngeal microbiomes of 23 AH patients, we observed similar trends in bacterial compositions. The enlargement of adenoids during early childhood, driven by immune mechanisms, may lead to chronic inflammation that spreads to adjacent mucosa and the oropharynx [19,20]. To explore the impact of surgical treatment, we compared preoperative and postoperative samples and found significant differences in microbiome compositions on the basis of β diversity analyses. Notably, we observed a decrease in Streptococcus and an increase in Neisseria among opportunistic pathogens postsurgery. Streptococcus, a common gram-positive coccus known for causing pus-forming infections, was reduced, whereas Neisseria, a gram-negative diplococcus with a unique cell wall structure rich in lipopolysaccharides, was increased [21,22]. The administration of broad-spectrum antibiotics such as cefaclor, which is more potent against gram-positive bacteria, during the perioperative period may partially explain these shifts in the microbial communities [23]. This might account for the variations in the microbiome between the two groups. More studies are needed to identify the microbiome of the oropharynx in AH patients.In this field, adopting effective antibiotic treatment against specific pathogens is crucial for successful patient outcomes. Chronic inflammation of adenoids, often triggered by pathogens such as bacteria, plays a crucial role in the development of adenoid hypertrophy. Frequent upper respiratory infections or exposure to environmental irritants may exacerbate the condition, further contributing to chronic inflammation and subsequent enlargement. By addressing the underlying causes of chronic inflammation, healthcare providers can help alleviate symptoms and improve overall respiratory health in affected individuals.Several limitations must be acknowledged in our study. First, while 16S rRNA gene sequencing is a powerful tool for identifying complex microbial communities, it primarily offers genus-level resolution, limiting comparisons with species-level data from culture-based studies. Second, the absence of a control group comprising healthy children hinders our ability to fully interpret the observed changes. Third, the small sample size impacts the statistical robustness of our findings. Additional challenges include potential contamination during sample handling, the lack of exploration of bacteria?host interactions, and the inability to detect viral pathogens. Future research should address these limitations by enrolling larger patient cohorts, including healthy controls, and incorporating assessments of potential interactions between microorganisms and their hosts. With the rapid evolution of sequencing technologies, achieving species level identification may soon become feasible. Our study underscores the microbiota diversity in the oropharynx of hospitalized children with AH and demonstrates significant alterations in microbiome composition following adenoidectomy and perioperative management. Further investigations are warranted to elucidate the clinical implications of these changes in the oropharyngeal flora.
CONCLUSION
Adenoidectomy and perioperative antibiotic administration in pediatric patients undergoing surgery for AH significantly alter the oropharyngeal microbiota. These findings underscore the substantial impact of surgical procedures and associated management strategies on the microbial communities residing in the oropharynx. Future studies with larger patient populations are needed to explore the clinical outcomes associated with these changes in the oropharyngeal flora.
REFERENCES
- Fossum CC, Chintakuntlawar AV, Price DL, Garcia JJ. Characterization of the oropharynx: Anatomy, histology, immunology, squamous cell carcinoma and surgical resection. Histopathology. 2017; 70: 1021-1029.
- Arambula A, Brown JR, Neff L. Anatomy and physiology of the palatine tonsils, adenoids, and lingual tonsils. World J Otorhinolaryngol Head Neck Surg. 2021; 7: 155-160.
- Niedzielski A, Chmielik LP, Mielnik-Niedzielska G, Kasprzyk A, Bogus?awska J. Adenoid hypertrophy in children: A narrative review of pathogenesis and clinical relevance. BMJ Paediatr Open. 2023; 7: e001710.
- Ahmad Z, Krüger K, Lautermann J. Adenoid hypertrophy-diagnosis and treatment: The new S2k guideline. HNO 2023; 71: 67-72.
- Zuliani G, Carron M, Gurrola J, Coleman C, Haupert M, Berk R, et al. Identification of adenoid biofilms in chronic rhinosinusitis. Int J Pediatr Otorhinolaryngol. 2006; 70: 1613-1617.
- Rajeshwary A, Rai S, Somayaji G, Pai V. Bacteriology of symptomatic adenoids in children. N Am J Med Sci. 2013; 5: 113-118.
- Johnston JJ, Douglas R. Adenotonsillar microbiome: An update. Postgrad Med J. 2018; 94: 398-403.
- Rogers GB, Wesselingh S. Precision respiratory medicine and the microbiome. Lancet Respir Med. 2016; 4: 73-82.
- Le TM, Rovers MM, van Staaij BK, van den Akker EH, Hoes AW, Schilder AG. Alterations of the oropharyngeal microbial flora after adenotonsillectomy in children: A randomized controlled trial. Arch Otolaryngol Head Neck Surg. 2007; 133: 969-972.
- Zhang X, Li X, Xu H, Fu Z, Wang F, Huang W, et al. Changes in the oral and nasal microbiota in pediatric obstructive sleep apnea. J Oral Microbiol. 2023; 15: 2182571.
- Brook I. Effects of antimicrobial therapy on the microbial flora of the adenoids. J Antimicrob Chemother. 2003; 51: 1331-1337.
- Dirain CO, Silva RC, Collins WO, Antonelli PJ. The adenoid microbiome in recurrent acute Otitis Media and Obstructive Sleep Apnea. J Int Adv Otol. 2017; 13: 333-339.
- Goeringer GC, Vidi? B. The embryogenesis and anatomy of Waldeyer’s ring. Otolaryngol Clin North Am. 1987; 20: 207-217.
- Lee CH, Hsu WC, Ko JY, Yeh TH, Lin MT, Kang KT. Revision adenoidectomy in children: A meta-analysis. Rhinology. 2019; 57: 411-419.
- Brook I, Shah K, Jackson W. Microbiology of healthy and diseased adenoids. Laryngoscope. 2000; 110: 994-999.
- Abranches J, Zeng L, Kajfasz JK, Palmer SR, Chakraborty B, Wen ZT, et al. Biology of Oral Streptococci. Microbiol Spectr. 2018; 6: 10.1128/ microbiolspec.gpp3-0042-2018.
- Rajeshwary A, Rai S, Somayaji G, Pai V. Bacteriology of symptomatic adenoids in children. N Am J Med Sci. 2013; 5: 113-118.
- Liang Y, Jiang Y, Wang F, Wen C, Deng Y, Xue K, et al. Long-term soil transplant simulating climate change with latitude significantly alters microbial temporal turnover. ISME J. 2015; 9: 2561-2572.
- Richtsmeier WJ, Shikhani AH. The physiology and immunology of the pharyngeal lymphoid tissue. Otolaryngol Clin North Am. 1987; 20: 219-228.
- Bogaert D, Keijser B, Huse S, Rossen J, Veenhoven R, van Gils E, et al. Variability and diversity of nasopharyngeal microbiota in children: A metagenomic analysis. PLoS One. 2011; 6: e17035.
- Engholm DH, Kilian M, Goodsell DS, Andersen ES, Kjærgaard RS. A visual review of the human pathogen Streptococcus pneumoniae. FEMS Microbiol Rev. 2017; 41: 854-879.
- Quillin SJ, Seifert HS. Neisseria gonorrhoeae host adaptation and pathogenesis. Nat Rev Microbiol. 2018; 16: 226-240.
- Meyers BR. Cefaclor revisited. Clin Ther. 2000; 22: 154-166.