Correlation between Obesity, Audio Vestibular and Retinal Disorders in OSAS
- 1. Department of Neuroscience Imaging and Clinical Sciences, Gabriele D’Annunzio University of Chieti-Pescara, Italy
- 2. Department of Surgery, Hashemite University, Jordan
- 3. Department of Unit of Human and Clinical Nutrition, Medical, Oral and Biotechnology Sciences, Gabriele D’Annunzio University of Chieti-Pescara, Italy,
- 4. Department of Medicine and Aging Sciences, Gabriele D’Annunzio University of Chieti Pescara, Italy
- †. These authors have contributed equally to this work and share first authorship
Abstract
Background: The inner ear is poorly vascularised and can be damaged by hypoxia related to old age, obesity and sleep apnea syndrome (OSAS). It is possible that these factors are also the basis of retinal pathologies.
Materials and methods: In this retrospective study, 20 OSAS-overweight patients and 20 healthy individuals were compared to assess the association between the audio-vestibular and retinal alterations and the degree of OSAS. All Subjects were subjected to polysomnography, morphometric evaluation, audiometry, vestibular potentials (C-VEMPs, O-VEMPs), Video-HIT and retinal evaluation.
Results: Regarding audio-vestibular disorders, statistically significant differences were found between OSAS patients and normal subjects on all the parameters considered. No retinal evaluation differences were observed.
Discussion: Obesity and OSAS can affect auditory and vestibular function proportionally with both the degree of OSAS and obesity. This correlation can be prevented with apneas treatment and the avoidance of obesity control and its risk factors editable.
Conclusion: Our results highlight that audio-vestibular screening of obese and OSAS patients in the early stage also suggests microvascular alterations and, therefore, a greater cardiovascular risk, better than retinal evaluation; this risk could be reduced by early treatment for OSAS and obesity.
Keywords
• OSAS
• VEMPs
• HIT
• Obesity
• Audiometry
• Retina
• Cardiovascular risk
Citation
Neri G, Iacovitti CM, Khasawneh L, Neri L, Gammone MA, et al. (2025) Correlation between Obesity, Audio-Vestibular and Retinal Disorders in OSAS. Ann Otolaryngol Rhinol 12(3): 1361.
INTRODUCTION
Obesity is linked with very serious health risks and when it is severe increase the risk of complications, such as coronary heart disease and end-stage renal disease. In the 2020 report of U.S. Department of health and human services of Centers for Disease Control and Prevention, a significantly increasing trend in obesity was observed. The age-adjusted prevalence of obesity among U.S. adults in 2017-2018 was from 40,0% among younger adults to 42.8 among older adults aged 60 and over % and from 1999–2000 through 2015–2016 [1]. A decrease in hearing ability in adults, particularly in the elderly, and the dizziness it is quite common and affects 16 to 25% of the elderly in relation to age [2], but the studies on the association between obesity, audio-vestibular changes and retinal disorders are limited and contradictory. In the last years in literature some Authors have highlighted the association between obesity and hearing loss both in subjects of advanced age [3-6], and in younger patients [7,8]. Others have highlighted the correlation between the fall event with obesity and dizziness [9-11]. Still others have linked retinal vascular changes with obesity [12,13], demonstrating their statistical association and finally, is universally know that the fundus oculi is an direct indicator of microcirculation injure. There is a linear correlation between obesity and OSAS. Patients with OSAS appear to be particularly prone to weight gain, compared to equally obese subjects without OSAS, in fact one of the main components contributing to sleep apnea is obesity and the chronic sleep apnea can lead to obesity because of increased serum ghrelin levels and decreased serum leptin levels [14]. These changes in serum leptin levels as well as leptin-receptor insensitivity could be involved in the pathogenesis of progressive obesity in OSAS. Leptin can reduce appetite and simultaneously increase respiratory drive in animal models; in humans, fasting leptin levels in patients with OSAS decreased with continuous positive airways pressure (CPAP) treatment [15]. In obese people, fat deposits in the upper respiratory tract and a decrease in muscle activity in this region, leading to hypoxic and apneic episodes, ultimately resulting in sleep apnea. These hypoxia/apnea episodes lead to a decrease in oxygen that is available in body tissues and blood vessels greatly contributing to the onset of atherosclerosis, the main risk factor for Cardiovascular Diseases (CVD) [16,17]. In 2013, Hwang demonstrated the presence of microvascular changes in the cochlear basal gyrus of obese mice [18]. This microvascular involvement, from which both audio-vestibular and retinal disorders derive, could have at the base a chronic hypoxia, present both in obese patients and in patients with OSAS, whose incidence in obese subjects is very high [19,20]. For these reasons, therefore deepening the relationship between obesity, osas, retinal and vestibular manifestations could throw new light on very common pathologies with very similar pathophysiological implications on the microcirculation. Aim of this study is to verify the possibility that vestibular loss is a direct indicator of cardiovascular risk [21].
MATERIAL AND METHODS
Subjects
We conducted a retrospective study on 40 subjects, 20 OSAS belonging to the OSAS center of the SS Annunziata Hospital of Chieti and 20 healthy patients. Of the study group patients, 5 were female and 15 males, aged between 30 and 80 (mean 59.3). Ten of these had received a diagnosis of obesity and 10 of overweight according to the NHLBI criteria [22], in relation to sex and age. All the subjects included were subjected to anamnestic collection and from the ENT visit. During the anamnesis, we took into account possible risk factors: smoking; male gender; degree of drowsiness; soft tissue laxity of the VAS, a parameter that was assessed by performing a fibro-laryngoscopy with Müller manoeuvre. All subjects gave their informed consent for inclusion before they participated in the study. The study was conducted in accordance with the Declaration of Helsinki of 1975 revised in 2013.
Exclusion criteria
We have considered also possible alterations which, depending on the case, may represent factors of aggravation and / or exclusion: tinnitus; average ear infections; severe neurological or psychiatric disease; brain tumor or vestibular schwannoma; diabetes mellitus; chronic renal failure; heart failure; chronic liver disease; hypertension and hyperlipidemia; previous ear surgery; history of acoustic trauma; exposure to ototoxic substances; professional noise exposure; autoimmune diseases; cancer; radiation exposure to the head and neck; pregnancy; current drug therapies.
Exams
All Patients were subjected to:
- Nocturnal Cardiorespiratory Monitoring (polysomnography)
The severity of OSAS was based on night cardiorespiratory monitoring data by means of the AHI (Apnea-Hypopnea Index) score, gold standard for the diagnosis of OSAS. In our sample we considered an AHI value equal to or less than 5 normal. The exam was performed using Philips Respironics Alice PDx
- Audiometry
The liminal tonal audiometric examination was performed using the LABAT audiometer (Audiolab model) carrying out the measurement of hearing by sending pure sound stimuli to different intensities and frequencies (125, 250, 500, 1k, 1.5k, 2k, 3k, 4k, 6k, 8k Hz) first in one ear, then in the other.
- Vestibular Evoked Myogenic Potentials (O-VEMPs and C-VEMPs)
Using LABAT Epic Plus model both, cervical and ocular VEMPs, where recorded using as acoustic stimulus the LOGON (short train of six cycles) at 500 Hz with a duration of 10 ms, with a frequency of four repetitions/sec and with an intensity of 130 dB, presented binaurally by air.
- C-VEMPs we used this test to evaluate the function of the saccular macula placed on the same side. During the acoustic stimulus, the patient was asked to push with the forehead towards the palm of the hand of the examiner. The signals were recorded on sternocleidomastoid muscle (SCM) searching three components were sought: a positive peak that appears at 13.3 ms (P13), a negative peak that occurs at 23 ms (N23) and the response amplitude.- O-VEMPS (Ocular-VEMPs): we used this test to evaluate the function of the utricular macula placed on the opposite side. During the acoustic stimulus the patient was asked to look upwards and fix a hypothetical target, placed at about 25-30°. The signals were recorded on the lower oblique and the lower rectum muscles of the eye, the potential peak was sought around 10 ms (N10); the amplitude was calculated automatically.
- Video-Head Impulse Test (V-HIT)
The V-HIT was fundamental to complete the study of the vestibular function, given its ability to investigate the presence or absence of corrective saccades, a further sign of vestibular dysfunction, in particular of the semicircular canals. This examination was carried out with OTOMETRICS ICS Impulse instrumentation, with glasses equipped with a camera capable of following the pupil (high speed USB camera - 250 Hz - for recording eye movements and identifying saccades). The study of oculomotricity required the use of a laser generator, a light target, an operator to impose passive head movements and a system for recording and analyzing eye movements (videonystagmography). At the end of this examination, the evaluation of all the semicircular canals on the right and left side, (lateral, anterior and posterior canal - LARP/ RALP) was obtained as the gain of the VOR (vestibular oculomotor reflex), given by the ratio between “eye speed” / “speed of the head “(expressed in degrees per second - o / sec), normally equal to 1.
- Morphometry
In collaboration with the staff of the Human and Clinical Nutrition Unit of the G. D’Annunzio University of Chieti, height, body weight, waist circumference and hip circumference (HC) of each patient were measured after a night fasting. Body mass index (BMI) was calculated as weight in kilograms divided by the square of height in meters (kg/m2) in order to define normal weight (BMI= 18.5-24.9 kg/m2), overweight (BMI= 25-29.9 kg/m2), and obesity (BMI ≥ 30 kg/m2). The WHR (waist-to-hip ratio) was calculated as waist measurement divided by hip measurement (both expressed in cm). WHR is used as a measurement of obesity, which in turn is a possible indicator of increased metabolic and cardiovascular risk. The WHO states that abdominal obesity is defined as a WHR above 0.90 for males and above 0.85 for females. The BAI (body adiposity index) was calculated as 100 x [(HC in cm/ (height in meters)1.5) -18], to calculate the percentage of fat mass with a validated error of 5%.
- Retinal examination.
In collaboration with the National Center of High Technology in Ophthalmology of Chieti, OCT-A (Optical Coherence Tomography Angiography of the optic disc, peri-papillary retina and macula), examination and photo acquisition of fundus oculi was performed on all subjects to identify vascular and retinal structural alterations.
RESULTS
This study was performed as an observational, longitudinal prospective (cohort) study. Being a purely exploratory study, a sample size calculation was not performed but we referred to the number of pieces reported in other studies and superimposable on ours. The quantitative variables were expressed with the calculation of the Average +/- standard deviation (SD), while the data are described in relative percentage frequency. The degree of association between the qualitative variables is expressed through the Pearson R index; the correlation between the data is represented graphically through scatter plot with trend line. The significant differences found in the evaluation were evaluated with the “t-Student” test, the level of statistical significance was set for p <0.05. Materials and Methods should be described with sufficient details to allow others to replicate and build on the published results.
Audiometry
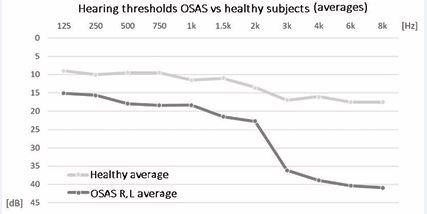
We found an increase in the auditory threshold on high frequencies in 38% of subjects affected by OSAS and in 5% of cases there was an increase in the auditory threshold on low frequencies, while in 57% of the subjects there was no alteration hearing. In the group of patients with increased hearing threshold, 62% were obese (of which 20% with severe obesity). Statistically significant increase in the hearing threshold were found in the OSAS patient group compared to the healthy control group (Table 1).
Table 1: Statistical results of the comparison of the hearing thresholds between OSAS.
|
Hearing thresholds |
Cases (OSAS) |
Controls (healthy) |
p |
|
3 KHz (dB) |
36 ± 24 |
17 ± 5 |
p<0,05 |
|
4 KHz (dB) |
38 ± 25 |
18 ± 8 |
p<0,01 |
|
6 KHz (dB) |
40 ± 25 |
18 ± 11 |
p<0,01 |
|
8 KHz (dB) |
40 ± 26 |
19 ± 10 |
p<0,01 |
The reduction of hearing was above all on the high frequencies (3-4-6-8 kHz) (Figure 1).
Figure 1 Comparison between the means of the hearing thresholds of OSAS patients compared to healthy subjects.
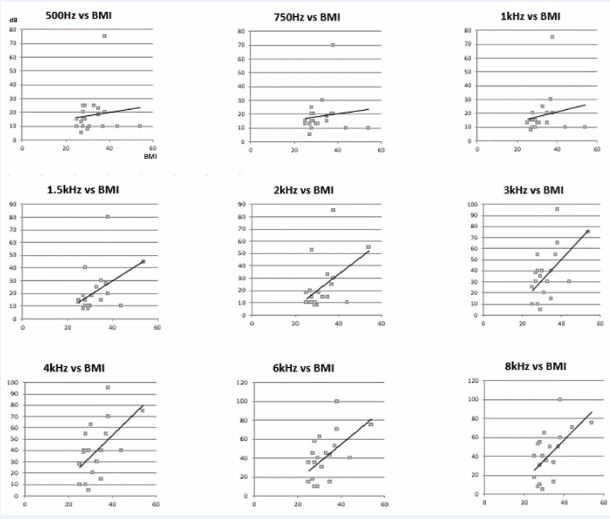
Statistically significant differences were found in the comparison of the thresholds hearing loss between obese OSAS patients and overweight OSAS patients also (Table 2, Figure 2).
Figure 2 Graphs of correlation between audiometric frequencies and BMI, in succession from low to high frequencies.
Table 2: Statistical results of the comparison of the hearing thresholds between obese OSAS patients and overweight OSAS patients.
|
Hearing thresholds |
OSAS (Obese) |
OSAS (Overweight) |
p |
|
1.5 KHz (dB) |
28 ± 22 |
15 ± 9 |
p<0,05 |
|
3 KHz (dB) |
46 ± 27 |
25 ± 16 |
p<0,01 |
|
4 KHz (dB) |
50 ± 27 |
28 ± 17 |
p<0,01 |
|
6 KHz (dB) |
53 ± 25 |
29 ± 17 |
p<0,01 |
|
8 KHz (dB) |
55 ± 26 |
31 ± 19 |
p<0,01 |
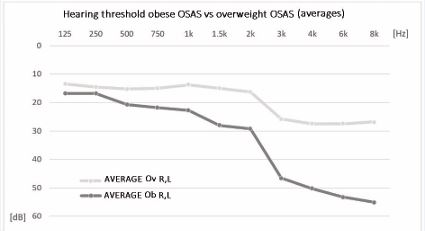
From the study of correlations, the direct dependence between the increase in the auditory thresholds and the various morphometric parameters taken into consideration (BMI, WHR, BAI) is evident (Table 3), in particular with the BMI (Figure 3).
Figure 3 Comparison of the hearing threshold averages of obese OSAS patients compared to overweight OSAS patients.
Table 3: Statistical results of the comparison between OSAS patients and healthy individuals.
|
|
Cases (OSAS) |
Controls (healthy) |
p |
|
C-VEMPs P13 (ms) |
13,9 ± 3 |
11,9 ± 1,4 |
p<0,05 |
|
C-VEMPs N23 (ms) |
20,3 ± 3,5 |
17,7 ± 2,9 |
p<0,05 |
|
C-VEMPs Amplitude |
12,9 ± 7,9 |
21,9 ± 6,9 |
p<0,01 |
|
O-VEMPs N10 (ms) |
11,7 ± 2,7 |
9,1 ± 0,8 |
p<0,01 |
|
O-VEMPs Amplitude |
3,4 ± 4,5 |
10,2 ± 6,4 |
p<0,01 |
|
V-HIT CSL |
0,9 ± 0,2 |
1,1 ± 0,2 |
p<0,05 |
Similar results to the previous ones have also been obtained with the comparison between the auditory thresholds and the degree of OSAS (AHI). From a further comparison it was also possible to identify an inverse correlation between the degree of physical activity (assessed in METs) and the audiometric thresholds.
Vestibular tests
Cervical VEMPs: Statistical analysis shows a significant delay in the appearance of the P13 wave (13.9 ms) in OSAS patients compared to that of control cases (11.9 ms), as well as among obese OSAS patients (P13 14.9 ms) and overweight (P13 12.9 ms) Similar speech for the N23 wave, whose average value in the OSAS (20.3 ms) is significantly greater (p <0.05) than that of the population of control (17.7 ms), as well as between obese (N23 21.4 ms) and overweight (N23 19.2 ms) OSAS patients. C-VEMPs amplitude analysis: in OSAS subjects we observed a significant reduction (p <0.05) of the amplitude of the potential, which on average was equal to 12.9 μV compared 21.9 μV in healthy subjects; also among obese OSAS patients we observed a significant reduction (p <0.05) of the amplitude of the potential (8.9 μV) compared to overweight OSAS patients (13.8 μV) (Table 4,5).
Table 4: Statistical results of the comparison between obese OSAS patients and overweight OSAS patients.
|
|
Cases (Obese OSAS) |
Controls (Overweight OSAS) |
p |
|
C-VEMPs P13 (ms) |
14,9 ± 3 |
12,9 ± 2,6 |
p<0,05 |
|
C-VEMPs N23 (ms) |
21,4 ± 3,4 |
19,2 ± 3,2 |
p<0,05 |
|
C-VEMPs Amplitude |
8,9 ± 5,6 |
13,8 ± 8,9 |
p<0,05 |
|
O-VEMPs N10 (ms) |
12,6 ± 2,7 |
10,9 ± 2,4 |
p<0,05 |
|
O-VEMPs Amplitude |
1,9 ± 3,5 |
4,9 ± 4,9 |
p<0,05 |
|
V-HIT CSL |
0,9 ± 0,2 |
1 ± 0,2 |
p<0,01 |
|
V-HIT CSP |
0,96 ± 0,2 |
1,1 ± 0,3 |
p<0,05 |
|
V-HIT RALP |
0,93 ± 0,2 |
1 ± 0,2 |
p<0,05 |
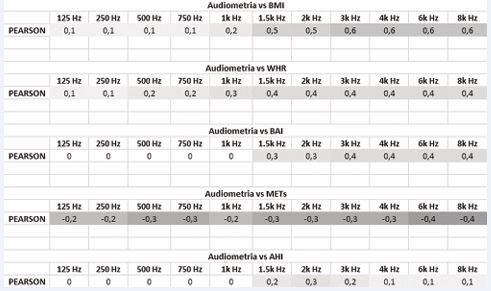
Table 5 : Correlation data, expressed by Pearson’s r index, between audiometric hearing thresholds and the other variables identified (BMI, WHR, BAI, METs, AHI).
Ocular VEMPs: The evaluation focused on the value of N10 and that of amplitude. We observed a significant delay (p <0.05) in the appearance of N10 in OSAS patients, which stood at an average value of 11.7 ms compared to 9.1 ms found in control cases; equivalent speech for obese OSAS patients (N10 12.6 ms) compared to overweight (N10 10.9 ms). The amplitude of the biphasic complex was significantly (p <0.05) reduced in OSAS patients; in these the mean value was 3.4 μV, compared to 10.2 μV of the control patients. Among obese OSAS patients, the amplitude was on average 1.9 μV, a value here too significantly (p <0.05) compared to overweight OSAS patients, with an average amplitude of 4.9 μV. In three OSAS subjects out of a total of 20 (15%), there was no bilateral potential (O-VEMPs) (Table 4,5).
Video-Head Impulse Test: From the comparison between OSAS subjects and healthy subjects, the average gain in OSAS patients tends to be lower than the value of the control cases, but the significance of the compared data was found only for the lateral semicircular canal, whose value average is 0.9 in OSAS subjects and 1.1 in healthy subjects. On the other hand, between the obese OSAS patients, compared to the overweight, a significant difference (p <0.05) was found between the means of the gains both for the lateral semicircular canal (OSAS obese 0.9; OSAS overweight 1) and for the posterior (OSAS obese 0.96; OSAS overweight 1.1), and also also for the RALP functional group (OSAS obese 0.93; OSAS overweight 1) (Table 4,5).
Morphometry: From the comparison between the different calculated morphometric variables and the results obtained from the vestibular-cochlear tests, various correlations of varying degrees were found, highlighted by the Pearson r-index. In particular, the increases in the hearing thresholds were directly proportional to the BMI, in particular in the high frequencies (Figure 2). The same BMI was significantly correlated to the variations recorded in the V-HIT (Table 6).
Table 6 Correlation data, expressed by Pearson’s r index, between the VOR gains obtained by the V-HIT and the other variables identified (BMI, WHR, BAI, METs, AHI).
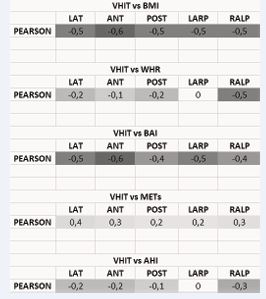
With regard to VEMPS, on the other hand, the correlation with the WHR was more significant (Figure 4, Figure 5). Finally, a direct correlation between the morphometric variables (in particular BMI and BAI) and the indicative values of the OSAS level (AHI) was highlighted (Figure 6). In all these correlations, by comparing the patient’s degree of activity (METs), an inverse proportion was found.
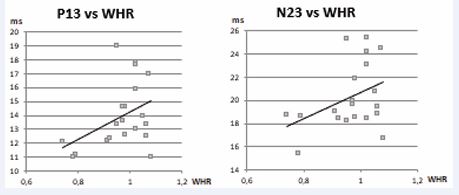
Figure 4 Correlation between the severity of obesity and the alterations found in the C-VEMPS traces. The blue dots represent the values of P13 and N23 found on the C-VEMPS traces and correlated to the WHR value that was quantified for each patient on the basis of the polysomnographic report. The trend line is shown in black, witnessing the 0.4 obtained with the calculation of the Pearson index which confirms the association between the delay in the appearance of the potential and the WHR index.
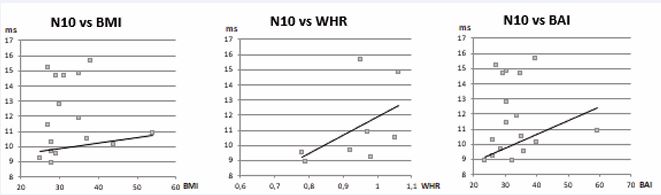
Figure 5 Correlation between the alterations found in the O-VEMPs traces and morphometric indices. The blue dots represent the N10 values correlated with BMI, WHR and BAI. The straight line highlights a general correlation between the delay of N10 and a higher value of BMI, WHR and BAI.
Retinal study: From the analysis of the data obtained through the study of the fundus, no statistically significant data were found (p <0.05).
DISCUSSION
The correlation between OSAS, obesity and inner ear disorders is currently being studied, as the emergence of obstructive pathology during sleep has opened up new scenarios and new fields of research, especially in the ENT field. Several studies have night apneas with metabolic syndrome linked [14-24], hyperleptinemia and resistance to leptin [25]. These results are important in order to identify a possible cause of the functional damage found in OSAS and obese patients. Leptin is a hormone produced by adipocytes, fundamental in regulating weight and with suppressive action of the appetite at the hypothalamic level. OSAS patients have leptin plasma levels 50% higher than similarly obese non-OSAS controls, therefore a state of leptin resistance has been hypothesized. A probable cause of this hyperleptinemia in OSAS patients is hypoxic sympathetic hyperactivity which leads to a reduction in the physiological inhibition of leptin secretion by down regulation of adipocytes’ beta-3 (β-3) receptors [26]. Since basal leptin levels predict the development of metabolic syndrome, OSAS hyperleptinemia could therefore also represent a mechanism of progression towards the metabolic syndrome itself. Leptin resistance can be a direct cause of weight gain, followed by all other obesity-related complications, including microvascular and functional alterations found in previous studies [3-13]. In fact, obesity is characterized by a marked endothelial dysfunction evidenced in several vascular districts including peripheral microcirculation, where a decreased nitrico oxide (NO) availability is detected: in this scenario, perivascular adipose tissue (PVAT) generates pro-inflammatory cytokines, including TNF-α which promotes superoxide generation in the vascular wall, with a well-demonstrated direct vascular detrimental effect. PVAT-derived pro-inflammatory cytokines represent an important source of low-grade inflammation and oxidative stress, which contribute to endothelial dysfunction characterizing obese patients [27].In previous studies, obesity was specifically placed in a causal relationship with possible microvascular alterations of the cochlear structure, with consequent functional damage expressed by hearing loss on high frequencies, without however investigating what the underlying causes of these modifications may actually be.
The linkage between all these alterations, in patients with OSAS and obesity, could be the hypoxia that apnoeic episodes would cause during sleep, especially in obese individuals, therefore with a greater cardiovascular risk. In a similar context, the ear, an organ with terminal vascularization, devoid of collateral circulation and therefore tending to be already hypoxic, would inexorably precipitate the efficiency of its vascular network, accusing damage that it could find, in the hearing disorder or in the disorder balance, its clinical manifestation. To better frame the nature of the damage that could affect the inner ear, it is useful to define the difference between an acute and / or chronic vascular event. An acute vascular event affecting the inner ear causes more or less extensive damage and specific symptoms. In OSAS, the characteristic is represented by short and intermittent episodes of chronic hypoxemia, followed by re-oxygenation cycles with consequent alternation between hypo perfusion and reperfusion until the development of ROS mediated oxidative stress. The organism tries to react and counter chronic hypoxia by implementing compensation mechanisms, for example through the increase of erythropoiesis mediated by Hypoxia Inducible Factor (HIF 1 and 2) [28,29], aimed at slowing the progression of the damage and the decline in the audio-vestibular function. Being a chronic damage, it is often underestimated or undiagnosed.The diagnostic process began with the audiometric examination, to look for signs of cochlear suffering. Audiometry was conducted on each patient, investigating both high and low frequencies and what emerged confirms what is reported in the scientific literature: a significant percentage of the OSAS population has a hearing reduction especially on high frequencies (3k, 4k, 6k, 8k Hz), particularly in the obese population. In the studied population, hearing damage was present in 38% of OSAS subjects and in 5% of cases there was an increase in the hearing threshold on low frequencies, while in 58% of the subjects there was no auditory alteration. In the group of patients with increased hearing threshold, 62% were obese (of which 20% with severe obesity). The damage to the auditory system, in our opinion, would derive from the cyclic apnoea-induced cochlear suffering which, with the aforementioned perfusion and reperfusion mechanisms, would result in microcirculatory endothelial damage, especially evident in patients with an already suffering microcirculation due to the obesity. From the study of the correlations the direct dependence between the increase of the auditory thresholds and the various morphometric parameters taken into consideration (BMI, WHR, BAI) is evident, in particular with the BMI (Figure 2, Table 3), similar results were also obtained with the comparison with the degree of OSAS (AHI) (Table 5). From a further comparison it was also possible to identify an inverse correlation between the degree of physical activity (assessed in METs) and the audiometric thresholds (Table 3).The values C-VEMPs, O-VEMPs and V-HITs obtained were validated through the t-Student and with the calculation of the Pearson index r. The analysis of the traces of C-VEMP on both sides was performed, the result was a trend that tends to delay in the appearance of the action potential. This trend was evidenced by P13 and N23 values temporally delayed both in OSAS patients compared to the average values of the healthy ones, both in overweight OSAS patients compared to obese OSAS patients (Tables 4 and 5).
In addition to the potential delay, in the C-VEMPs tracing we also found a morphological alteration, given that the amplitude of the wave was also reduced on average. This delay and the statistically significant amplitude reduction (p <0.05), are certainly an expression of the macular receptor suffering following hypoxic damage, which in the vestibular apparatus produces more marked damage even with slight desaturations. To verify a possible correlation between the altered C-VEMPs traces and the severity of OSAS and obesity, a statistical analysis was performed to correlate the severity of OSAS (expressed with the AHI score) and obesity (BMI, WHR, BAI) with all the parameters obtained. In particular, a correlation was observed as a function of WHR with both P13 and N23 (Figure 4).Also in the O-VEMPs there was a difference in the average indicative values for the appearance of the potential: The N10 appears on average delayed in the subjects affected by OSAS, and in particular among the obese. By comparing the values of N10 with the calculated morphometric values (BMI, WHR, BAI), the Pearson r-index showed a positive correlation in all 3 cases (Figure 5).
The Video-HIT examination, studied statistically with Il t-Student, on the values referred to the lateral semicircular canal showed a reduced average value for OSAS patients compared to the average value of the control group, but no significance was found in the comparison of the values for LARP and LARP, where p> 0.05. The lateral canal is in fact the most important in the utricle-canal complex and is the one that manages the direction on the horizontal plane together with the utricle. It is therefore no wonder that, in the face of a greater need for metabolic demand, it is the one that manifests the most signs of suffering in relation to hypoxia. In the same comparison, carried out between the obese OSAS patients and overweight OSAS patients, significant differences resulted not only on the CSL (lateral semicircular canal), but also on the CSP (posterior) and on the RALP functional group (Table 5). In particular, from the calculation of the Pearson r index, it was evident (Table 6): the direct correlation between the increase in the gain of the VOR and the various anthropometric indices (BMI and BAI in particular); the direct correlation between the increase in the gain of the VOR and the degree of OSAS (AHI index); an inverse correlation between the increase in VOR gain and the degree of physical activity (assessed in METs). The various morphometric indices (BMI, WHR, BAI, METs) calculated, were found to be in direct correlation with the audio-vestibular alterations assessed and with the degree of OSAS (Figure 6).
Regarding the ophthalmological study of retinal vascularization, although it represents a reliable index of microvascular damage in the literature, in the population examined we did not found statistically significant clinical alterations of impact (p <0.05). From this we can deduce that the visual system responds to a greater extent to the vascular deficits of ischemia reperfusion, so deficiencies or situations of chronic hypoxia were, in this case, better assessable with otoneurological investigations which, ultimately, can be sensitive and predictive investigations of microvascular damage, especially if associated with morphometric assessments. The difference in behaviour between these districts is partly anatomically explainable because the ophthalmic artery calibre is about of 2 mm in diameter [30,31], while the average caliber of the internal auditory artery is 0.19 mm (range 0,15-0,24 mm), [32] and partly from the fact that the inner ear is particularly vulnerable to ischemia due to its high energy expenditure guaranteed only by a terminal artery with little collaterals Damage resulting from hypoxia is therefore less tolerated by the auditory system than by the visual system. In our opinion, this is the most important data we have found as for the first time we have the possibility to predict the damage resulting from osas and obesity even before the damage is in such an advanced state that it can be observed on the retina. For this reason, the inner ear can rightly be considered the sentinel of the cerebral microcirculation.
CONCLUSIONS
The hypoxic suffering that results from a history of OSAS inevitably damages the districts sprayed with under dense and non-anastomosed vascular networks. It is easy for the inner ear to accuse the damage that results from chronic hypoxia, but since these alterations are all expression of chronic damage and not immediately giving evident clinical signs, they are often underestimated or undiagnosed. These disorders, even if subclinical, over time can lead to much more evident and irreversible outcomes. For this reason it is important to intervene when the damage is still early, an objective that can be achieved by identifying clusters of patients who present multiple risk factors at the same time (obesity, OSAS), and starting them on a standardized procedure of diagnostic tests and therapies. The first signs of suffering can be sought by evaluating both auditory and vestibular function with specific investigations. This study confirmed the possibility that the preventive evaluation of groups of patients with audio-vestibular and obese disorders could allow an early diagnosis of OSAS. At the same time, it also placed attention on the opposite consideration, namely that in patients suffering from both nocturnal and obese apnoea, an early assessment of the audio-vestibular function may be useful to identify such alterations as early as possible [33-35]. In addition, it is also necessary to start treatment for sleep apnoea and obesity (when indicated) as soon as possible, in order to remove as many risk factors as possible to the development of disabling audio-vestibular changes and cardiovascular complications.
CONFLICT OF INTEREST
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
AUTHOR CONTRIBUTIONS
G. Neri: Conceptualization, Methodology, Software L Khasawneh, N. D’Orazio Data curation, Writing- Original draft preparation. Iacovitti, L.Neri: Visualization, Investigation. CM Iacovitti, A Gammone,, : Supervision.: G.Neri, N. d’Orazio, L. Agnifili: Writing- Reviewing and Editing: L Corradi, K. Xhepa.
ACKNOWLEDGMENTS
This is a short text to acknowledge the contributions of specific colleagues, institutions, or agencies that aided the efforts of the authors.
REFERENCES
- Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of Obesity and Severe Obesity Among Adults: United States, 2017-2018. NCHS Data Brief. 2020; 1-8.
- Löhler J, Walther LE, Hansen F, Kapp P, Meerpohl J, Wollenberg B, et al. The prevalence of hearing loss and use of hearing aids among adults in Germany: a systematic review. Eur Arch Otorhinolaryngol. 2019; 276: 945-956.
- Kim TS, Park SW, Kim DY, Kim EB, Chung JW, So HS. Visceral adipose tissue is significantly associated with hearing thresholds in adult women. Clin Endocrinol (Oxf). 2014; 80: 368-375.
- Hwang JH, Wu CC, Hsu CJ, Liu TC, Yang WS. Association of centralobesity with the severity and audiometric configurations of age-related hearing impairment. Obesity (Silver Spring). 2009; 17: 1796-1801.
- Hwang JH, Hsu CJ, Liu TC, Yang WS. Association of plasma adiponectin levels with hearing thresholds in adults. Clin Endocrinol (Oxf). 2011; 75: 614-620.
- Croll PH, Voortman T, Vernooij MW, Baatenburg de Jong RJ, Lin FR, Rivadeneira F, et al. The association between obesity, diet quality and hearing loss in older adults. Aging (Albany NY). 2019; 11: 48-62.
- Üçler R, Turan M, Garça F, Acar ?, Atmaca M, Çankaya H. The association of obesity with hearing thresholds in women aged 18-40 years. Endocrine. 2016; 52:46-53.
- Lalwani AK, Katz K, Liu YH, Kim S, Weitzman M. Obesity is associated with sensorineural hearing loss in adolescents. Laryngoscope. 2013; 123: 3178-3184.
- Kim SY, Kim MS, Sim S, Park B, Choi HG. Association Between Obesity and Falls Among Korean Adults: A Population-Based Cross-Sectional Study. Medicine (Baltimore). 2016; 95: e3130.
- Corna S, Aspesi V, Cau N, Scarpina F, Valdés NG, Brugliera L, et al. Dizziness and falls in obese in patients undergoing metabolic rehabilitation. PLoS One, 2017; 12: e0169322.
- Lin HW, Bhattacharyya N. Impact of dizziness and obesity on the prevalence of falls and fall-related injuries. Laryngoscope. 2014; 124: 2797-2801.
- Gishti O, Jaddoe VW, Hofman A, Wong TY, Ikram MK, Gaillard R. Body fat distribution, metabolic and inflammatory markers and retinal microvasculature in school-age children. The Generation R Study. Int J Obes (Lond). 2015; 39: 1482-1487.
- Liccardo D, Mosca A, Petroni S, Valente P, Giordano U, Mico’ AG, et al. The association between retinal microvascular changes, metabolic risk factors, and liver histology in pediatric patients with non- alcoholic fatty liver disease (NAFLD). J Gastroenterol. 2015; 50: 903- 912.
- Chaput JP, Després JP, Bouchard C, Tremblay A. Short sleep duration is associated with reduced leptin levels and increased adiposity: Results from the Quebec family study. Obesity (Silver Spring). 2007; 15: 253-261.
- Harsch IA, Konturek PC, Koebnick C, Kuehnlein PP, Fuchs FS, Pour Schahin S, et al. Leptin and ghrelin levels in patients with obstructive sleep apnoea: effect of CPAP treatment. Eur Respir J. 2003; 22: 251- 257.
- Chen X, Pensuksan WC, Lohsoonthorn V, Lertmaharit S, Gelaye B, Williams MA. Obstructive Sleep Apnea and Multiple Anthropometric Indices of General Obesity and Abdominal Obesity among Young Adults. Int J Soc Sci Stud. 2014; 2: 89-99.
- Jehan S, Zizi F, Pandi-Perumal SR, Wall S, Auguste E, Myers AK, et al. Obstructive Sleep Apnea and Obesity: Implications for Public Health. Sleep Med Disord. 2017; 1: 00019.
- Hwang JH, Hsu CJ, Yu WH, Liu TC, Yang WS. Diet-induced obesity exacerbates auditory degeneration via hypoxia, inflammation, and apoptosis signalling pathways in CD/1 mice. PLoS One. 2013; 8: e60730.
- Lechner M, Breeze CE, Ohayon MM, Kotecha B. Snoring and breathing pauses during sleep: interview survey of a United Kingdom population sample reveals a significant increase in the rates of sleep apnoea and obesity over the last 20 years - data from the UK Sleep Survey. Sleep Med. 2019, 54: 250-256.
- Bozkurt NC, Beysel S, Karbek B, Unsal ?O, Cakir E, Delibasi T. VisceralObesity Mediates the Association Between Metabolic Syndrome and Obstructive Sleep Apnea Syndrome. Metab Syndr Relat Disord. 2016; 14: 217-221.
- Lee CC, Ho HC, Su YC, Chiu BC, Su YC, Lee YD, et al. Increased risk of vascular events in emergency room patients discharged home with diagnosis of dizziness or vertigo: a 3-year follow-up study. PLoS One. 2012; 7: e35923.
- Gibbons GH. Managing Overweight and Obesity in Adults: Systematic Evidence Review from the Obesity Expert Panel NHLBI Edition. 2013.
- Vgontzas AN, Bixler EO, Chrousos GP. Sleep apnea is a manifestation of the metabolic syndrome. Sleep Med Rev. 2005; 9: 211-224.
- Drager LF, Togeiro SM, Polotsky VY, Lorenzi-Filho G. Obstructive sleep apnea: a cardiometabolic risk in obesity and the metabolic syndrome. J Am Coll Cardiol. 2013; 62: 569-576.
- Berger S, Polotsky VY. Leptin and Leptin Resistance in the Pathogenesis of Obstructive Sleep Apnea: A Possible Link to Oxidative Stress and Cardiovascular Complications. Oxid Med Cell Longev. 2018; 2018: 5137947.
- Phillips BG, Kato M, Narkiewicz K, Choe I, Somers VK. Increases in leptin levels, sympathetic drive, and weight gain in obstructive sleep apnea. Am J Physiol Heart Circ Physiol. 2000; 279: H234-H237.
- Virdis A, Masi S, Colucci R, Chiriacò M, Uliana M, Puxeddu I, et al. Microvascular Endothelial Dysfunction in Patients with Obesity. Curr Hypertens Rep. 2019; 21: 32.
- Labarca G, Gower J, Lamperti L, Dreyse J, Jorquera J. Chronic intermittent hypoxia in obstructive sleep apnea: a narrative review from pathophysiological pathways to a precision clinical approach. Sleep Breath. 2020; 24: 751-760.
- Nath B, Szabo G. Hypoxia and hypoxia inducible factors: diverse roles in liver diseases. Hepatology. 2012; 55: 622-633.
- Hedges TR. Ophthalmic artery blood flow in humans. Br J Ophthalmol.2002; 86:1197.
- Lang J, Kageyama I. The ophthalmic artery and its branches, measurements and clinical importance. Surg Radiol Anat. 1990; 12: 83-90.
- Haidara A, Peltier J, Zunon-Kipre Y, N’da HA, Drogba L, Gars DL. Microsurgical Anatomy of the Labyrinthine Artery and Clinical Relevance. Turk Neurosurg. 2015; 25: 539-543.
- Kim HA, Lee H. Recent Advances in Understanding Audiovestibular Loss of a Vascular Cause. J Stroke. 2017; 19: 61-66.
- Lee H, Kim JS, Chung EJ, Yi HA, Chung IS, Lee SR, et al. Infarction in the territory of anterior inferior cerebellar artery: spectrum of audiovestibular loss. Stroke. 2009; 40: 3745-3751.
- Kim JS, Lee H. Inner ear dysfunction due to vertebrobasilar ischemic stroke. Semin Neurol. 2009; 29: 534-540.