Effectiveness Comparison of Three Budesonide Brands in Adults with Allergic Rhinitis: A Prospective Randomized Clinical Trial
- 1. Federal University of Santa Catarina, Brazil
- 2. State University of Londrina, Brazil
- 3. University of Chicago, USA
Abstract
Objective: To compare the efficacy of topical nasal budesonide brands available in Brazil to treat allergic rhinitis (AR).
Methods: An open-label, prospective, superiority randomized clinical trial was conducted involving patients with a confirmed diagnosis of moderate-severe AR. Fifty-seven individuals were randomized into three groups. Each group underwent a 4-week treatment cycle with one of the three brands of topical nasal budesonide: Budecort Acqua® (brand name), Busonid® (brand name) and Noex® (generic). Each patient was submitted to olfactory function tests (University of Pennsylvania Smell Identification Test, UPSIT®), nasal obstruction questionnaire (Nose Obstruction Symptom Rating Scale, NOSE), Peak Nasal Inspiratory Flow (PNIF), and the Rhinitis Control Assessment Test (RCAT) before and after treatment.
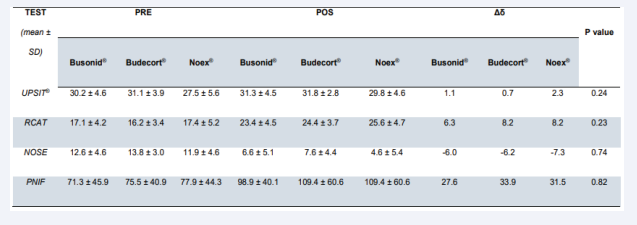
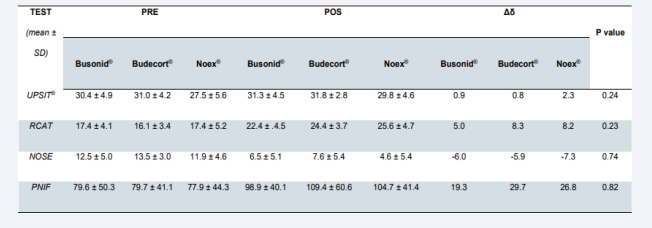
Results: Nineteen patients received Budecort Acqua®, 19 Busonid®, and 19 Noex®. Of the 57 randomized patients, 50 returned for data collection after 4 weeks of treatment. The number of dropouts was not statistically significant among the groups (p=0.13). All tests UPSIT®, PNIF, NOSE, and RCAT, significantly improved after 4 weeks of intervention in the three groups studied (p<0.01). None of the tests showed a statistically significant difference when compared among the groups, both pre- and post-treatment values, in both ITT and PP data analyses, UPSIT® (p=0.24 and p=0.26), PNIF (p=0.83 and p=0.79), NOSE (p=0.74 and p=0.58) and RCAT (p=0.23 and p=0.14). No serious adverse effects were reported in any of the three groups analyzed by the present study.
Conclusion: This study showed that the generic form of nasal budesonide and two corresponding brand drugs have similar efficacy in treating AR. Further trials are required to compare the long-term effectiveness and safety of generic and brand-name medications.
Keywords
• Allergi rhinitis
• Corticosteroid
• Clinical trial
• Olfactory function test
• Nose scale
CITATION
Mathias LB, Trindade LFG, de Moraes LS, Scussiatto HO, Garcia ECD, Fornazieri MA, et al. (2024) Effectiveness Comparison of Three Budesonide Brands in Adults with Allergic Rhinitis: A Prospective Randomized Clinical Trial. Ann Otolaryngol Rhinol 11(2): 1334.
INTRODUCTION
The gold standard treatment for allergic rhinitis (AR) is topical corticosteroids [1], due to their safety profile and effectiveness in the control of inflammation of the nasal mucosa [2]. Because of that, many brands of topical corticosteroids have been developed and are widely used by a large number of patients with AR [3].
For approval by the National Health Surveillance Agency (ANVISA), the regulatory agency of Brazil’s Ministry of Health, generic drugs for topical use do not require bioavailability quantification tests. The requirements are restricted to proof of the drug’s presence, the same concentration concerning the reference drug, and the same excipients in comparable concentrations [4]. Despite the rules stipulated by this regulatory agency, complaints about the lesser clinical effect of generic drugs in daily medical practice are not rare [5]. There is only one study in the literature comparing brands of topical budesonide in AR patients, which demonstrated difference in efficacy in favor of the brand-name drug. However, this study was not a randomized clinical trial and used subjective and non-validated methods to analyze the symptomatic response and complications of the proposed treatments [6].
Because of the limited evidence in the literature, there is prejudice and mistrust in prescribing generic medications5 and their real effectiveness remains uncertain. We conducted a randomized clinical trial, using validated methods, to demonstrate whether there are differences among the brands of topical nasal budesonide in the effectiveness of the treatment of AR.
MATERIAL AND METHODS
Subjects
Fifty-seven patients were randomized in blocks, aged between 17 and 55 years, with a diagnosis of moderate-severe AR, based on compatible clinical data (nasal obstruction, runny nose, sneezing, nasal itching) and confirmed by specific serum IgE or Prick Test (skin allergy test).
We excluded patients who did not complete high school to reduce the bias in the questionnaire scores. The following exclusion criteria were also included: pregnant and breastfeeding women, history of anosmia, Parkinson’s disease, Alzheimer’s disease, schizophrenia, and epilepsy. Women who scored lower than 18 on University of Pennsylvania Smell Identification Test (UPSIT®) and men who scored lower than 16 were excluded, assuming they have anosmia and therefore olfactory function could not be assessed after treatment [7,8]
Randomizantion, Interventions and Blinding: Fifty- seven patients were randomly distributed into 3 groups. The randomization was in blocks of nine. Each drug was delivered to patients by a collaborator not aware of the study objectives. Each group underwent a 4-week treatment cycle of one of the three brands of topical nasal budesonide currently available in the local market: 19 patients with Budecort Acqua® (brand- name), 19 with Busonid® (brand-name) and 19 with Noex® (generic). Regardless of the brand, the recommended dose was 64mcg in each nostril, twice a day. Each patient was submitted, before and after treatment, to olfactory testing and nasal obstruction and symptom assessment questionnaires: Rhinitis Control Assesment Test (RCAT), Nasal Obtruction Symptom Rating Scale (NOSE), Peak Nasal Inspiratory Flow (PNIF) and UPSIT®. In addition, each patient received a medication usage diary to document the doses used and adverse effects. The record of drug use for less than 22 complete days was considered an exclusion criterion for the present study.
Specific Formulas of The Three Brands of Nasal Budesonide: In addition to the presence of budesonide as the main component, each drug studied has a specific formula. In the case of Budecort Acqua® 64mcg (AstraZeneca®), each mL of medication contains 1.28mg of budesonide, sodium chloride, polysorbate 80, microcrystalline cellulose, carmellose sodium, disodium edetate, propylene glycol, hydrochloric acid, sodium hydroxide, and distilled water. Each mL of Busonid® 64mcg (Aché®) contains 1.28mg of budesonide, microcrystalline cellulose, carmellose sodium, glucose, polysorbate 20, disodium calcium edetate dihydrate, purified water, hydrochloric acid, sorbitan trioleate, freon 11 and freon 12. Lastly, each mL of Noex® 64mcg (Eurofarma®) contains 1.28mg of budesonide, sodium chloride, polysorbate 80, microcrystalline cellulose, carmellose sodium, disodium edetate, propylene glycol, hydrochloric acid, sodium hydroxide, and distilled water.
Questionnaires and Other Instruments of Evaluation: All patients were evaluated at baseline and after 4 weeks with questionnaires and instruments to evaluate the olfactory function and the control of symptoms of AR. First of all, a general questionnaire was applied to evaluate the general profile of the patient, adherence to treatment, symptoms related to the use of medication, and use of other drugs during the research. Second, the olfactory function was assessed by the University of Pennsylvania Smell Identification Test (UPSIT®), which is considered the gold standard in olfactory assessment. The test is carried out by the patient and consists of 40 aromas exhaled after scraping a tape located in a test notebook. From the score achieved, it is possible to objectively classify the quality of the patient’s smell [9].
Finally, the degree of nasal obstruction and the severity of symptoms of AR was assessed by the application of the Peak Nasal Inspiratory Flow (PNIF), the Rhinitis Control Assessment Test (RCAT), and the Nasal Obstruction Symptom Rating Scale (NOSE). The PNIF is a portable test, easy to apply and of low maintenance cost, which allows the assessment of nasal airflow resistance, reflecting the level of obstruction the patient presents [10]. The RCAT consists of a simple, quick, and self-administered six-question questionnaire about the patient’s nasal and allergic symptoms, which occurred in the last week, and was developed to assess the control of rhinitis [11-13]. The last test applied was the NOSE. It is a simple, quick, and self-administered questionnaire with five questions related to nasal obstruction and its repercussions, which occurred in the last month, being developed to assess their impact on the patient’s quality of life. Furthermore, it can discriminate between patients with and without nasal obstruction [14-16].
Ethical Aspects and Biosafety: The drugs administered to the sample have been widely available on the local market for many years and have already been extensively studied and analyzed previously. The treatment of AR with topical budesonide, in addition to being one of the most conventional, as explained in previous literature, presents a low rate of side effects, due to its low systemic absorption. The results disclosed in this text completely preserve the identities of the participating patients.
The present study was approved by the Ethics Committee for Research Involving Human Beings (opinion number: XXXXX) and each patient signed a consent form after being properly instructed on the methods and purposes of the study. The Brazilian Registry of Clinical Trials (REBEC) is XXXXX.
Statistical Analysis: Sample size calculation was based on a four-point difference in the RCAT at the end of treatment, a four- point standard deviation, with a significance level of 5% and a power of 80% for a superiority clinical trial. Seventeen patients were needed to find the difference among the groups. To avoid significant sample losses, the subgroups were increased to 19 patients each.
The normality of the quantitative variables was verified by the Shapiro-Wilk test and the Levene’s test was applied to check the homoscedasticity. Then, Analysis of variance ANOVA (complemented by Tukey’s test) and Kruskal-Wallis were used for data analysis for comparison purposes among groups. The significant results were those with a value of p<0.05, with a 95% confidence interval. Statistical analysis was performed using SPSS software (version 20; IBM Corp., Armonk, NY).
RESULTS
Seventy-two patients with AR were recruited between April 2019 and May 2021. Of these, we excluded 15 patients due to exclusion criteria: use of antibiotics, antihistamines, oral corticosteroids, and vasoconstrictors in the last 30 days (n=6), history of nasal surgery (n=1), history of head trauma (n=3), asthma (n=3) and presence of nasal polyposis on nasofibroscopy in the first evaluation (n=1). In addition, one patient was excluded for not having symptoms of rhinitis. Thus, 57 patients were randomized, 19 in the Budecort Acqua® group, 19 in the Busonid® group and 19 in the Noex® group. Of the 57 randomized patients, 50 returned for data collection after 4 weeks of treatment. Seven patients did not return after 4 weeks of treatment for post- intervention data collection. Per protocol (PP) data analysis was performed for the 50 patients who completed the study and intention to treat (ITT) data analysis for a total of 57 randomized patients.
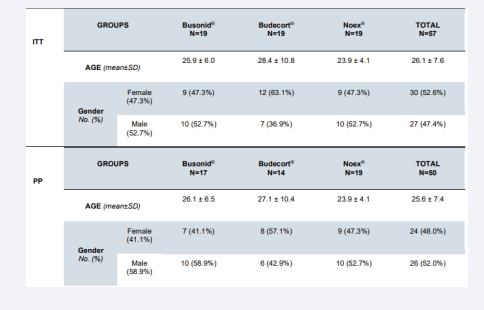
The median age was 26 in ITT and 25 in PP data analysis. The minimum age was 17 and the maximum was 54 and there was no statistical difference concerning age among the groups in ITT (p=0.21) and PP (p=0.84) data analysis respectively. Female patients were 52.6% (30/57) in ITT and 48% (24/50) in PP data analysis and both there was no statistical difference regarding gender (p=0.17 and p=0.48 respectively) among the groups (Table 1). The number of dropouts was not statistically significant among the groups (p=0.25). The age and gender were not statistically different among groups (p=0.84 and p=0.24 respectively).
Table 1: Demographic data of the patients in the trial.
On ITT data analysis, UPSIT®, RCAT, NOSE, and PNIF scores followed a normal distribution (p=0.56; p=0.68; p=0.74 and p=0.08 respectively) and on PP data analysis, the values of RCAT (p=0.48) and NOSE (p=0.45) were normally distributed.
NOSE AND RCAT
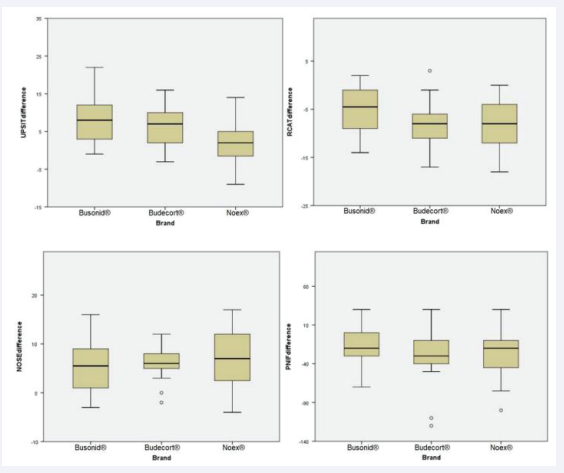
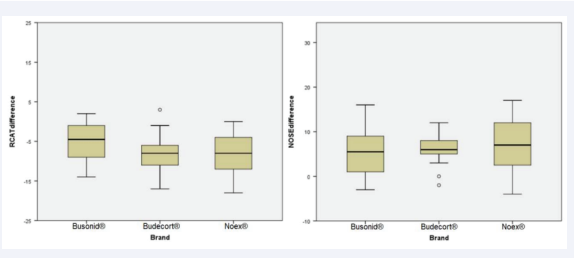
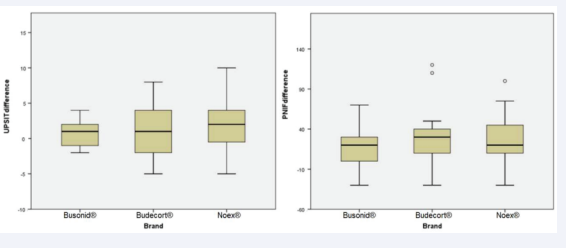
The impact of nasal congestion on quality of life, as well as rhinitis symptom control, were evaluated respectively by the nasal obstruction symptom assessment scale (NOSE) and by the rhinitis control assessment test (RCAT), which are validated questionnaires. Both scores showed significant improvement after 4 weeks of treatment in the three studied groups (p<0.01, x=-6.63, 95% CI -7.93, -5.32 and p<0.01, x=7.45, 95% CI 6.10, 8.80, respectively). There was no significant difference between the NOSE scores of the three groups before treatment and after treatment in the ITT [Figure 1, Table 2] and PP [Figure 2, Table 3] analyses, considering the ANOVA (p=0.74 and p=0.58, respectively). Similarly, no divergence was found in RCAT scores between the 3 treatment groups in the ITT [Figure 1, Table 2] and PP [Figure 2, Table 3] analyses, using the ANOVA (p=0.23 and p=0.14, respectively).
Figure 1: Boxplot graph comparing the means of pre- and post-treatment differences among 3 brands of nasal budesonide (Busonid®, Budecort® and Noex®), when University of Pennsylvania Smell Identification Test (UPSIT®), Rhinitis Control Assesment Test (RCAT), Nasal Obtruction Symptom Rating Scale (NOSE) and Peak Nasal Inspiratory Flow (PNIF) tests were evaluated by ANOVA, in intention-to-treat (ITT) data analysis
Table 2: Results of pre- and post-treatment tests when comparing different brands of intranasal budesonide in intention-to-treat (ITT) data analysis.
Figure 2: Boxplot graph comparing the means of pre- and post-treatment differences among 3 brands of nasal budesonide (Busonid®, Budecort® and Noex®), when Rhinitis Control Assesment Test (RCAT) and Nasal Obtruction Symptom Rating Scale (NOSE) tests were evaluated by ANOVA, in per-protocol (PP) data analysis
UPSIT®
The University of Pennsylvania Smell Identification Test (UPSIT®) achieved significant improvement after treatment of the three tested nasal topical budesonide brands (p<0.01, x =1.56, 95% CI 0.67, 2.45). There was no significant difference between the groups, both in pre-treatment and post-treatment values, in both ITT [Figure 1, Table 2] and PP [Figure 3, Table 3] analyses, using ANOVA and Kruskal-Wallis (p=0.24 and p=0.26, respectively).
Figure 3: Boxplot graph comparing the means of pre- and post-treatment differences among 3 brands of nasal budesonide (Busonid®, Budecort® and Noex®), when University of Pennsylvania Smell Identification Test (UPSIT®) and (Peak Nasal Inspiratory Flow) PNIF tests were evaluated by Kruskal-Wallis, in per-protocol (PP) data analysis.
Table 3: Results of pre- and post-treatment test when comparing different brands of intranasal budesonide in per-protocol (PP) data analysis
PNIF
The peak nasal inspiratory flow (PNIF) showed significant improvement after 4 weeks of treatment in the three groups evaluated (p<0.01, x=25.09, 95% CI 16.70, 33.48). Analyzing the groups among themselves, there was no significant difference between pre- and post-treatment values in the ITT [Figure 1, Table 2] and PP [Figure 3, Table 3] analyses, ANOVA and Kruskal- Wallis (p=0.83 and p=0.79, respectively).
Adverse Effects
No serious adverse effects were reported in any of the three groups analyzed by the present study. There were three reports of epistaxis (one in each group) and one report of dysphonia in the Busonid® group. Overall, neither the frequency of reported adverse events nor the number of subjects who dropped out was significantly different between the Budecort Acqua®, Busonid® and Noex® groups.
DISCUSSION
The effects of different brands of budesonide on olfactory function and the symptoms and quality of life of patients with AR were similar. We did not find any significant difference in the scores of the questionnaires applied to this study. Due to the requirements of ANVISA on the brand and generic medications, we can assume that the overall effects of these drugs are clinically the same, despite the differences in the composition of the drugs [17,18]. Our results can also be due to the fact that bioequivalence of a generic drug must be evaluated by appropriate randomized controlled clinical trials, in addition to bioavailability studies, ensuring the non-inferiority of the generic drug to the reference in terms of efficacy and safety [19-25].
Intranasal corticosteroids are the most effective therapeutic agents for AR, as demonstrated by the findings of three meta- analyses, and are equal to or superior to the combination of an antihistamine and an antileukotriene [26-29]. They act by decreasing the inflammatory cell response, inhibiting the release of cytokines, reducing mucus production and decreasing the response of leukotrienes and prostaglandins. There are many formulations available, and none have been shown to be superior in safety or efficacy [30]. Budesonide is the only agent that has an FDA category B pregnancy classification [31]. Despite their universally accepted effectiveness, inhaled steroids pose risks to patients. The most commonly reported adverse effects are headache, throat irritation, epistaxis, burning and nasal dryness [32]. Skeletal growth rate was unaffected in children treated with mometasone for 1 year in a randomized placebo-controlled trial [33].
We conducted the statistical analyses with our olfactory and allergy data, one using ANOVA and Kruskal-Wallis tests resulting in no divergence among groups of treatment. Despite the sample losses that occurred, there was still no difference among groups when we used the data of intention-to-treat and per-protocol analyses. All of this increases the robustness of our results and reinforces that the sample calculation is correct.
Although the excipients could have other effects, that could cause different side effects [34,35], we demonstrated that the excipients in the drugs neither play an important role in terms of controlling the symptoms of patients with AR nor cause diverse effects on olfactory function. We also find an improvement in UPSIT® in the patients submitted to treatment with budesonide. Our patients’ symptoms of AR improved with the use of nasal budesonide for 4 weeks, as measured by RCAT. The symptoms of nasal obstruction were reduced comparing the patients at the beginning and the end of the study, as proven by the NOSE test and the PNIF. These results are consistent with many previous studies in the literature, increasing the reliability of our outcomes [36-39].
Many previous studies were conducted using diverse types of drugs and found no clinical difference in patients treated with brand medications and generic ones. Maneechotesuwan et al., investigated the anti-inflammatory effects of salmeterol/ fluticasone combination, pulmonary inhalation medication, through sputum eosinophil count, and fraction of exhaled nitric oxide as primary and secondary endpoints, respectively. Results did not suggest any significant difference between generic and brand-name drugs in controlling asthma symptoms after a 30-day course of treatment [40,41]. A prospective cohort of 58 epileptic patients evaluated switching from brand-name levetiracetam to a generic one, as well as the incidence of reported adverse effects and loss of therapeutic control. No statistically significant differences were found in the number of adverse events or seizures in patients before switching to peer versus 6 months after switching, with an overall return of 3.4% of patients to brand-name drugs [42]. Similarly, using data from a retrospective crossover cohort study of 616 patients, no statistically significant increase in hospitalizations or emergency room visits was found after switching from brand-name lamotrigine to a generic one [43]. A recent prospective cohort study found no statistically significant differences in intraocular pressure for patients with glaucoma after switching from the brand-name dorzolamide- timolol eye drops to the generic [44].
We acknowledge we had a short follow-up duration and adding more time for follow-up could have changed the results. For that reason, longer-term studies are needed to provide information on the comparative efficacy of drugs in AR. It is important to note that the most relevant AR guidelines recommend that patients treated with nasal corticosteroids be reassessed in 4 to 12 weeks, and this study followed these guidance [45-47]. In addition, some patients did not return after treatment, requiring data imputation
Intranasal corticosteroids are the most effective therapeutic agents for AR, as demonstrated by the findings of three meta- analyses, and are equal to or superior to the combination of an antihistamine and an antileukotriene [26-29]. They act by decreasing the inflammatory cell response, inhibiting the release of cytokines, reducing mucus production and decreasing the response of leukotrienes and prostaglandins. There are many formulations available, and none have been shown to be superior in safety or efficacy [30]. Budesonide is the only agent that has an FDA category B pregnancy classification [31]. Despite their universally accepted effectiveness, inhaled steroids pose risks to patients. The most commonly reported adverse effects are headache, throat irritation, epistaxis, burning and nasal dryness [32]. Skeletal growth rate was unaffected in children treated with mometasone for 1 year in a randomized placebo-controlled trial [33].
We conducted the statistical analyses with our olfactory and allergy data, one using ANOVA and Kruskal-Wallis tests resulting in no divergence among groups of treatment. Despite the sample losses that occurred, there was still no difference among groups when we used the data of intention-to-treat and per-protocol analyses. All of this increases the robustness of our results and reinforces that the sample calculation is correct.
Although the excipients could have other effects, that could cause different side effects [34,35], we demonstrated that the excipients in the drugs neither play an important role in terms of controlling the symptoms of patients with AR nor cause diverse effects on olfactory function. We also find an improvement in UPSIT® in the patients submitted to treatment with budesonide. Our patients’ symptoms of AR improved with the use of nasal budesonide for 4 weeks, as measured by RCAT. The symptoms of nasal obstruction were reduced comparing the patients at the beginning and the end of the study, as proven by the NOSE test and the PNIF. These results are consistent with many previous
studies in the literature, increasing the reliability of our outcomes
[36-39].
Many previous studies were conducted using diverse types of drugs and found no clinical difference in patients treated with brand medications and generic ones. Maneechotesuwan et al., investigated the anti-inflammatory effects of salmeterol/ fluticasone combination, pulmonary inhalation medication, through sputum eosinophil count, and fraction of exhaled nitric oxide as primary and secondary endpoints, respectively. Results did not suggest any significant difference between generic and brand-name drugs in controlling asthma symptoms after a 30-day course of treatment [40,41]. A prospective cohort of 58 epileptic patients evaluated switching from brand-name levetiracetam to a generic one, as well as the incidence of reported adverse effects and loss of therapeutic control. No statistically significant differences were found in the number of adverse events or seizures in patients before switching to peer versus 6 months after switching, with an overall return of 3.4% of patients to brand-name drugs [42]. Similarly, using data from a retrospective crossover cohort study of 616 patients, no statistically significant increase in hospitalizations or emergency room visits was found after switching from brand-name lamotrigine to a generic one [43]. A recent prospective cohort study found no statistically significant differences in intraocular pressure for patients with glaucoma after switching from the brand-name dorzolamide- timolol eye drops to the generic [44].
We acknowledge we had a short follow-up duration and adding more time for follow-up could have changed the results. For that reason, longer-term studies are needed to provide information on the comparative efficacy of drugs in AR. It is important to note that the most relevant AR guidelines recommend that patients treated with nasal corticosteroids be reassessed in 4 to 12 weeks, and this study followed these guidance [45-47]. In addition, some patients did not return after treatment, requiring data imputation to analyze the results (intention-to-treat) and a longer follow-up duration would certainly increase losses, which could bias the results. Furthermore, the fact that this study was open-label for the evaluated individuals could be related to a possible bias.
CONCLUSIONS
The results of this trial showed no difference in the clinical efficacy of three topical nasal budesonide, one being a generic drug (Noex®) and the others considered brand-name drugs (Budecort Acqua® and Busonid®), in the treatment of AR, through validated tests and questionnaires. Considering that the symptoms of the disease and the quality of life were improved by the three products, the generic medication may be a decent option in treating patients with AR. However, future non- inferiority trials are needed to compare the long-term efficacy of generic and reference drugs.
REFERENCES
- Greiner AN, Hellings PW, Rotiroti G, Scadding GK. Allergic rhinitis. Lancet. 2011; 378: 2112–2122.
- Van Cauwenberge P, C Bachert, G Passalacqua, J Bousquet, G W Canonica, S R Durham, et al. Consensus statement on the treatment of allergic rhinitis. European Academy of Allergology and Clinical Immunology. Allergy, 2000, 55, 116-134.
- Bousquet J, Josep MAnto, Claus Bachert, Ilaria Baiardini, Sinthia Bosnic-Anticevich, G Walter Canonica, et al. Allergic rhinitis. Nature Reviews 2020; 6: 1-17.
- ANVISA. Resolution 391, of August 9, 1999. Approves the technical regulations for generic medicines. Official Gazette of the Union, Brasília, Aug 10. 1999. Section I, p.62.
- Shrank WH, Cox ER, Fischer MA, Mehta J, Choudhry NK. Patients ’perceptions of generic medications. Health Aff (Millwood). 2009; 28: 546–56.
- Gurdeep SM, Philip R, Rosalind S. Rhinocort vs Eltair: A comparative review of a patented and generic drug. Tropical Biomedicine. 2005; 22: 221–224.
- Doty RL. Treatments for smell and taste disorders: A critical review. Handb Clin Neurol. 2019; 164: 455-479.
- Marin C, Dolores Vilas, Cristobal Langdon, Isam Alobbid, Mauricio Lopez-Chacon, Antje Haehner, et al. Olfactory Dysfunction in Neurodegenerative Diseases. Current Allergy and Asthma Reports. 2018: 18: 42.
- Fornazieri MA,Clayson Alan dos santos, Thiago Freire Pinto Bezerra,Fabio de Rezende Pinna,Richard Louis Voegels, Richard L Doty . Development of normative data for the Brazilian adaptation of the university of pennsylvania smell identification test. Chemical Senses. 2015; 40: 141–149.
- Teixeira RUF, Carlos Eduardo Monteiro Zappelini, Fábio Silva Alves, Everardo Andrade da Costa. Peak nasal Inspiratory flow evaluation as an objective method of measuring nasal airflow. Brazilian Journal of Otorhinolaryngology. 2011; 77: 473–480.
- Schatz M, Eli O Meltzer, Robert Nathan, M Jennifer Derebery, Matthew Mintz, Richard H Stanford, et al. Psychometric validation of the rhinitis control assessment test: A brief patient-completed instrument for evaluating rhinitis symptom control. Annals of Allergy, Asthma and Immunology. 2010; 104: 118–124.
- Fernandes PH, Fausto Matsumoto, Dirceu Solé, Gustavo Falbo Wandalsen. Translation into Portuguese and validation of the Rhinitis Control Assessment Test (RCAT) questionnaire. Braz J Otorhinolaryngol. 2016; 82: 674–679.
- Meltzer EO. Allergic Rhinitis. Burden of Illness, Quality of Life, Comorbidities, and Control. Immunol Allergy Clin North Am. 2016; 36: 235–248.
- Stewart MG, David L Witsell, Timothy L Smith, Edward M Weaver, Bevan Yueh, Maureen T Hannley. Development and validation of the Nasal Obstruction Symptom Evaluation (NOSE) Scale. Otolaryngology- Head and Neck Surgery. 2004; 130: 157–163.
- Bezerra TFP, F G M Padua, R R M Pilan, M G Stewart, R L Voegels. Cross- cultural adaptation and validation of a quality of life questionnaire: the Nasal Obstruction Symptom Evaluation questionnaire. Rhinology. 2011; 49: 227–231.
- Lachanas VA, Stergiani Tsiouvaka, Malamati Tsea, Jiannis K Hajiioannou, Charalampos E Skoulakis. Validation of the Nasal Obstruction Symptom Evaluation (NOSE) Scale for Greek Patients. Otolaryngology -- Head and Neck Surgery. 2014; 151. 819–823.
- Dunne S, Shannon B, Dunne C, Cullen W. A review of the differences and similarities between generic drugs and their originator counterparts, including economic benefits associated with usage of generic medicines, using Ireland as a case study. BMC Pharmacology and Toxicology. 2013; 14: 1.
- Keith LG, Oleszczuk JJ, Stika CS, Stine S. Generics: what’s in a name?. Int J Fertil Womens Med. 1998; 43: 139-149.
- Beiraghdar F, Panahi Y, Einollahi B, Nemati E, Ghadiani MH, Sahebkar A, et al. Investigation of the efficacy of a biogeneric recombinant human erythropoietin alfa in the management of renal anemia in patients on hemodialysis: a multi-center clinical trial. Clinical Laboratory. 2012; 58: 737-45.
- Sayyah M, Argani H, Pourmand Gh, Amini H, Ahmadiani A. Pharmacokinetics, efficacy, and safety of iminoral compared with neoral in healthy volunteers and renal transplant recipients. Transplantation proceedings, 2007; 39: 1214-8.
- Kazemifard AG, Moore DE, Mohammadi A. Polarographic determination of benzaldehyde in benzyl alcohol and sodium diclofenac injection formulations. Journal of Pharmaceutical and Biomedical Analysis. 2002; 30: 257-62.
- Heshmat R, Taheri E, Larijani B. Comparison of a generic and a brand metformin products in type II diabetes: A double blind randomized clinical trial study. DARU Journal of Pharmaceutical Sciences. 2007; 15: 113-7.
- Aleyasin A, Hanafi S, Saffarieh E, Torkamandi H, Allahyari S, Sadeghi F, et al. Efficacy of generic granisetron vs Kytril for PONV in major gynecological operations: A Randomized, Double-blind Clinical Trial. Iranian Journal of Pharmaceutical Research. 2012; 11: 1059.
- Hadjibabaie M, Khoee SH, Nematipoor E, Gholami K, Fatahian A, Jahangard Z. Comparison of efficacy and tolerability of different brands of amlodipine in patients with mild to moderate hypertension. J Pharm Care. 2015; 1: 41-44.
- Namazi M, Yousefi Z, Shirazi M, Shaykholeslami M, Vakili H, Moatamedi M, et al. Comparison of the clinical efficacy of three brands of warfarin. Indian Journal of Pharmacology. 2004; 36: 360.
- Weiner JM, Abramson MJ, Puy RM. Intranasal corticosteroids versus oral H1 receptor antagonists in allergic rhinitis: systematic review of randomised controlled trials. BMJ. 1998; 317: 1624–29.
- Yãnez A, Rodrigo GJ. Intranasal corticosteroids versus topical H1 receptor antagonists for the treatment of allergic rhinitis: a systematic review with meta-analysis. Ann Allergy Asthma Immunol. 2002; 89: 479–84.
- Wilson AM, O’Byrne PM, Parameswaran K. Leukotriene receptor antagonists for allergic rhinitis: a systematic review and meta- analysis. Am J Med. 2004; 116: 338-44.
- Wilson AM, Orr LC, Sims EJ, Lipworth BJ. Effects of monotherapy with intra-nasal corticosteroid or combined oral histamine and leukotriene receptor antagonists in seasonal allergic rhinitis. Clin Exp Allergy. 2001; 31: 61–68.
- Okano M. Mechanisms and clinical implications of glucocorticosteroids in the treatment of allergic rhinitis. Clin Exp Immunol. 2009; 158: 164-73.
- Sur DK, Scandale S. Treatment of Allergic Rhinitis. Am Fam Physician. 2010; 81: 1440–6.
- Demoly P. Safety of intranasal corticosteroids in acute rhinosinusitis. Am J Otolaryngol. 2008; 29: 403–13.
- Schenkel EJ, Skoner DP, Bronsky EA, S D Miller, D S Pearlman, A Rooklin, et al. Absence of growth retardation in children with perennial allergic rhinitis after one year of treatment with mometasone furoate aqueous nasal spray. Pediatrics. 2000; 105: E22.
- Inoue Y, Kayoko Furuya, Miruto Matumoto, Isamu Murata, Masayuki Kimura, Ikuo Kanamoto. A comparison of the physicochemical properties and a sensory test of Acyclovir creams. International Journal of Pharmaceutics 2012; 436: 265– 271.
- Kahook MY, Robert D Fechtner, L Jay Katz, Robert J Noecker, David A Ammar. Stability of Generic Glaucoma Medications. Current Eye Research. 2012; 37: 101–108.
- Nguyen TP, Patel ZM. Budesonide irrigation with olfactory training improves outcomes compared with olfactory training alone in patients with olfactory loss. International Forum of Allergy & Rhinology, 2018; 8: 977-981.
- Fokkens WJ, Endre Cserháti, José Manuel Lopes dos Santos, Fatima Praca, Marinus van Zanten, Alexander Schade, et al. Budesonide aqueous nasal spray is an effective treatment in children with perennial allergic rhinitis, with an onset of action within 12 hours. Ann Allergy Asthma Immunol 2002; 89: 279–284.
- Devalia JL, D Prime, D H Richards. Effect of Variable Inspiratory Flow Rate on the Performance of the Budesonide Rhinocort Turbuhaler. Clin Drug Invest. 2001; 21: 195-201.
- Bende M, Teresa Carrillo, Ida Vóna, Maria Graça da Castel-Branco, Lars Arheden. A randomized comparison of the effects of budesonide and mometasone furoate aqueous nasal sprays on nasal peak flow rate and symptoms in perennial allergic rhinitis. Ann Allergy Asthma Immuno. 2002; 88: 617-23
- Maneechotesuwan K, Assawabhumi J, Rattanasaengloet K, Suthamsmai T, Pipopsuthipaiboon S, Udompunturak S. Comparison between the effects of generic and original salmeterol/fluticasone combination (SFC) treatment on airway inflammation in stable asthmatic patients. Journal of the Medical Association of Thailand. 2014; 97: S91-100.
- Panahi M, Ghanei H, Maghsoudi, Sara Saffar Soflaei, Amirhossein Sahebkar. Generic versus brand-name fluticasone/salmeterol. Acta Biomed. 2018; 89: 186-192.
- Vari MS, Pinto F, Mencaroni E, Giovanna Giudizioso, Carlo Minetti, Angela La Neve, et al. Safety of overnight switch from brand-name to generic levetiracetam. Clin Drug Investig. 2016; 36: 87–91.
- Hartung DM, Middleton L, Svoboda L, McGregor JC. Generic substitution of lamotrigine among medicaid patients with diverse indications: a cohort-crossover study. CNS Drugs. 2012; 26: 707–16.
- Kim YI, Kim JH, Lee TY, Lee KW. Efficacy and safety of glaucoma patients ’switch from a 2% dorzolamide/0.5% timolol fixed- combination brand-name drug to its generic counterpart. J Ocul Pharmacol Ther. 2015; 31: 335–9.
- Bousquet J, P Van Cauwenberge, N Khaltaev, Aria Workshop Group, World Health Organization. Allergic rhinitis and its impact on asthma. J Allergy Clin Immunol. 2001; 108: S147-334.
- Seidman MD, Gurgel RK, Lin SY, Seth R Schwartz, Fuad M Baroody, James R Bonner, et al. Clinical practice guideline: allergic rhinitis. Otolaryngol Head Neck Surg. 2015; 152: 197–206.
- Wheatley LM, Togias A. Allergic rhinitis. N Engl J Med. 2015; 372: 456-63.