Neoadjuvant Targeted Molecular Therapy Facilitates Surgery in Locally Advanced Thyroid Cancer
- 1. University of Sydney Endocrine Surgical Unit, Royal North Shore Hospital, Australia
- 2. Department of Endocrinology, Royal North Shore Hospital, Australia
- 3. Faculty of Medicine and Health, University of Sydney, Australia
- 4. Cancer Genetics, Kolling Institute of Medical Research, Royal North Shore Hospital, Australia
Abstract
Introduction: Locally advanced thyroid cancer presents significant therapeutic challenges, particularly when tumours exhibit aggressive biology and invade critical structures, rendering them unresectable at presentation. This study evaluates the efficacy of neoadjuvant multikinase inhibitor therapy in enhancing surgical resectability in patients with locally advanced disease.
Methods: A retrospective review was conducted on patients with locally advanced, unresectable thyroid cancer treated between January 2017 and December 2022. Multidisciplinary team assessment identified seven patients, of whom five received lenvatinib with three-monthly follow-up. Treatment response was evaluated using RECIST criteria, and outcomes included therapy safety, progression to surgery, and overall survival.
Results: Five patients with cT3/T4 disease were treated with neoadjuvant lenvatinib and achieved objective response to treatment with significant reduction in tumour size (mean 30.5 ± 17.4%). Four patients proceeded to surgery achieving a 50% R1 and 50% R2 resection rate. Adverse events were mild or moderate, and resolved with dose reduction or when neoadjuvant therapy was withheld pre-operatively. To date three patients who underwent surgery are alive with a median follow up of 44.7 months (range 30.1-53.3 months), with one death from distant disease 13 months after diagnosis.
Conclusion; In this small series, neoadjuvant lenvatinib therapy demonstrated effective antitumour activity in LATC and facilitated surgical resection.
Keywords
• Neoadjuvant
• Lenvatinib
• Adult
• Advanced Thyroid Cancer
• Multikinase Inhibitor
Citation
Liu LS, Pasch JA, Papachristos A, Wijewardene A, Clifton-Bligh RJ(2026) Neoadjuvant Targeted Molecular Therapy Facilitates Surgery in Locally Advanced Thyroid Cancer. Ann Otolaryngol Rhinol 13(1): 1380.
INTRODUCTION
Thyroid cancer is a common endocrine malignancy with approximately 4,100 cases diagnosed in Australia in 2023, accounting for 0.25 per cent of new cancer diagnoses [1]. Despite the increase in incidence, mortality rates remain less than 0.5 per 100 000, reflecting a generally favourable prognosis [1]. However, 2-4.1% of patients present with locally advanced thyroid cancer (LATC) with invasion of critical structures such as the trachea, oesophagus or major blood vessels [2-4]. LATC is commonly associated with hobnail and tall cell variants of papillary thyroid cancer (PTC), poorly differentiated thyroid cancer (PDTC), medullary thyroid cancer (MTC) and anaplastic thyroid cancer (ATC) [5,6]. Regardless of the histological type, LATC has an increased incidence of locoregional recurrence and distant metastases [7,8]. Amongst patients with LATC, local complications including tumour invasion causing airway obstruction, and massive haemorrhage from trachea-oesophageal fistula were amongst the most common causes of death [9].
Although primary surgical resection remains the cornerstone of LATC management, there is increased focus on reducing surgical morbidity and mortality. The role of tyrosine kinase inhibitors (TKI) in advanced thyroid cancer has evolved beyond adjuvant systemic therapy for metastatic disease [10,11]. Their use in the neoadjuvant setting to downstage the tumour in anticipation of surgical resection has marked a significant shift in the treatment approach for LATC [12]. Case reports of neoadjuvant therapy targeting molecular drivers including BRAF V600E, RET, NTRK and ALK show promise, enabling less extensive surgery, reduced incomplete resection, as well as providing important prognostic information through assessing pathological response to therapy [13-16].
Lenvatinib is a multikinase inhibitor (MKI) with anti-tumour activity used in the treatment of several cancers including differentiated thyroid cancer, renal cell carcinoma, melanoma and hepatocellular carcinoma [17]. Its broad anti-tumour and anti-angiogenic activity occur due to potent inhibition of phosphorylation and activation of tyrosine kinases including vascular endothelial growth factor receptors (VEGFR) 1-3, fibroblast growth factor receptors (FGFR) 1-4, platelet-derived growth factor receptor (PDGFR) alpha, c-KIT and the RET proto-oncogene [18,19]. The over-expression or activation of these signalling proteins in thyroid malignancy is associated with tumour proliferation and metastasis [20-23]. Since first use in 2017, lenvatinib has demonstrated efficacy in improving progression free survival in radioiodine refractory DTC, as well as MTC and ATC [24-27]. Despite the promising results of lenvatinib and other TKIs, neoadjuvant targeted therapy is not yet considered standard treatment for LATC. Further research is necessary to guide recommendations and real world changes to management. This study describes the experience of a quaternary referral centre in Australia with neoadjuvant lenvatinib in patients presenting with unresectable LATC.
METHODS
Case data were prospectively collected on five patients with LATC treated at a tertiary referral centre from January 2017 to December 2022. All cases were reviewed by the Endocrine Tumour Multidisciplinary Team (MDT), comprised of endocrinologists, endocrine surgeons, anatomical pathologists, radiologists, nuclear medicine physicians and radiation oncologists. Tumour imaging characteristics were assessed by three experienced endocrine surgeons. Patients identified as “surgically non resectable”, “resectable with unacceptable morbidity” or “inoperable due to poor pre-operative functional status” were considered for neoadjuvant MKI therapy. Immunohistochemistry and molecular testing were conducted to identify targetable mutations, including BRAF V600E, RET, NTRK and ALK, and patients who received neoadjuvant BRAF / MEK inhibitor therapy were excluded. Ethics approval was granted from the Northern Sydney Local Health District Human Research Ethics Committee (#2020/ETH02787) and participants provided informed consent.
Selected patients were commenced on 24mg of lenvatinib daily and monitored for potential side effects including hypertension (HTN), arrhythmias, proteinuria,renal dysfunction, fatigue, and bleeding. Mild or moderate adverse reactions were managed with dose reduction while interval breaks were considered in the event of severe side effects. Baseline imaging with high resolution contrast enhanced Computed Tomography (CT) and Positron Emission Tomography (PET) was performed, with CT repeated every three months. Patients were monitored by an endocrinologist and clinical nurse consultant with regular electrocardiograms and biochemical tests, including carcinoembryonic antigen (CEA) and calcitonin for patients with MTC. The MDT determined suitability and timing of surgical resection based on clinical evaluation and radiological response to treatment. If deemed resectable, preoperative laryngoscopy was performed to check vocal cord function.
The primary outcome measure was objective response rate (ORR) according to Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 [28]. Efficacy was defined as tumour reduction enabling surgical resection. If surgery was unable to be performed, neoadjuvant therapy was considered ineffective [29]. Secondary outcomes measures included completeness of resection R0 (complete), R1 (microscopic residual disease), R2 (macroscopic residual disease), overall survival and safety of therapy. Changes in CEA and calcitonin levels for patients with MTC were also recorded (Table 1).
Table 1: Patient demographics and tumour characteristics.
|
Case |
1 |
2 |
3 |
4 |
5 |
|
Age at presentation (years) / Sex |
76 / F |
66 / M |
76 / F |
66 / M |
76 / M |
|
ECOG |
1 |
1 |
2 |
0 |
1 |
|
Stage at diagnosis |
cT4b N1b M0 |
cT4b N0 M1 |
T3a N1b M1 |
T3a N1b M1 |
cT4b N1b M1 |
|
Histologic type |
ATC |
ATC |
MTC |
MTC |
PTC |
|
Genetic alteration |
BRAF WT, somatic PTEN and p53 alteration |
NRAS p.Gln61Arg and p.Thr50Ile (thyroid), TERT c.- 124C>T (liver) |
RET M918T |
RET |
BRAF V600E |
|
Reason for neoadjuvant therapy |
Vascular involvement |
Vascular and paravertebral muscle involvement |
Poor functional status due to ACTH dependent Cushings |
Presence of metastatic distant disease |
Vascular and tracheo- esophageal involvement |
|
Metastatic lesions |
None |
Lung, skeletal |
Lung, liver |
Lung, liver, skeletal |
Lung |
|
Pre-MKI biochemistry |
– |
– |
Calcitonin 958 CEA 340 |
Calcitonin 3090 CEA 275 |
– |
|
Pre-operative biochemistry |
– |
– |
Calcitonin 257 CEA 104.5 |
Calcitonin 299 CEA 64 |
– |
|
Latest biochemistry |
– |
– |
Calcitonin 59 CEA 14 |
Calcitonin <5 CEA 6 |
– |
|
EBRT |
Yes |
Yes |
No |
No |
Yes |
|
Adjuvant treatment |
Lenvatinib |
Lenvatinib, pembrolizumab |
Selpercatinib |
Lenvatinib, selpercatinib |
- |
|
Overall survival, status |
53.3 months, alive |
13 months, deceased |
30.1 months, alive |
44.7 months, alive |
6.5 months, deceased |
RESULTS
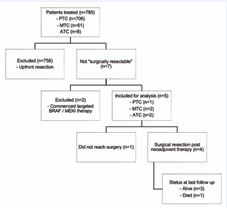
Between January 2017 and December 2022, a total of 706 PTC, 51 MTC and 8 ATC patients were treated surgically at our institution. Of these, three PTC (0.4%), two MTC (4%) and two ATC (25%) patients were assessed as not surgically resectable at initial presentation. Two PTC patients were commenced on combination BRAF / MEK inhibitor therapy, while the remaining five patients were commenced on neoadjuvant lenvatinib therapy (Figure 1). All patients presented with stage 4 disease with locoregionally advanced tumours, including involvement of adjacent vital structures (trachea, oesophagus, major vessels), bulky / matted lymphadenopathy or distant metastases (4 lung, 2 skeletal, 2 liver). Patient demographics as well as tumour and treatment characteristics are detailed in Table 1 and Table 2. Detailed case descriptions are presented in the Appendix, with patient 2 included in another case series [30].
Figure 1 Flowchart outlining management of patient cohort.
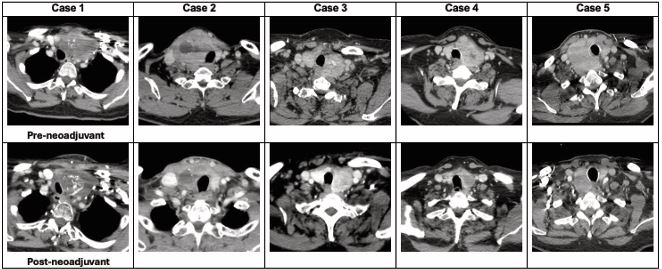
Histological types receiving neoadjuvant therapy included one PTC, two MTC and two ATC. Associated genomic mutations are presented in Table 1. Patients received a median of 5.5 months of neoadjuvant therapy (range 1.6-8.3). Three patients (60%) achieved a partial response and two had stable disease by RECIST 1.1 criteria (Figure 2). The mean best percentage change in the target lesion diameter was a 30.5 ± 17.4% decrease.
Table 2: Neoadjuvant therapy and operation details.
|
Case |
1 |
2 |
3 |
4 |
5 |
|
Duration of therapy (months) |
8.3 |
6.5 |
1.6 |
3 |
5.5 |
|
Side effects of TKI |
None |
HTN, fatigue, foot ulcers |
HTN, mouth ulcers, hand and foot syndrome |
None |
HTN, fatigue, oral ulcers |
|
RECIST * |
9.1% stable disease |
52.1% partial response |
17.5% stable disease |
40.9% partial response |
32.8% partial response |
|
Thyroidectomy |
Left hemithyroidectomy |
Right hemithyroidectomy |
Total thyroidectomy |
Total thyroidectomy |
N/A (progression distant disease) |
|
Neck dissection |
Bilateral CND |
Bilateral CND, R LND (III/IV) |
Bilateral CND, L LND (II- V), R LND (III/IV) |
Bilateral CND, L LND (II-V) |
- |
|
Sites of ETE |
Brachiocephalic vein / SVC, L RLN |
Skeletal muscle, trachea, R RLN |
Perithyroidal fat |
L RLN |
- |
|
Resection margin |
R2 |
R2 |
R1 |
R1 |
- |
|
Final histopathology |
Poorly differentiated (insular) carcinoma, >95% tumour necrosis |
ATC with >90% necrosis |
Multifocal MTC |
MTC with 50% tumour regression |
- |
*Change in longest diameter of target lesion.
Figure 2 Neck CT before and after neoadjuvant therapy with lenvatinib.
Despite local tumour response, the overall results for neoadjuvant lenvatinib were heterogeneous (Table 2). Four patients (80%) proceeded to surgery, whilst the PTC patient did not due to progression of distant metastatic disease. Among patients who underwent surgery, total thyroidectomy was performed for two patients with MTC, while both ATC patients underwent hemithyroidectomy. All patients underwent concurrent central neck dissection with lateral neck dissection performed for patients with involved lateral nodes. One patient required a hemi sternotomy to resect mediastinal disease and debulk tumour thrombus. No patients required resection of the recurrent laryngeal nerve (RLN), aerodigestive structures or vital vascular structures. No blood transfusion or tracheostomies were performed, and there were no major surgical complications. Lenvatinib was withheld for a median of 11.5 days (range 8-14) before surgery.
The most common adverse events (AE) were HTN (60%), oral ulcers (40%) and hand-foot syndrome (20%). Dose reduction was required for three patients (60%). Post operatively MKI therapy was continued with lenvatinib monotherapy (n=1), lenvatinib and pembrolizumab (n=1), or selpercatinib (n=2). 3-year overall survival was 60% with a median follow-up period of 30.1 months from diagnosis (range 6.5-53.3 months). To date, three patients are alive and despite evidence of persistent distant metastases on imaging, they remain free of locoregional disease. One patient died due to progressive distant metastases but demonstrated no locoregional recurrence post-operatively.
DISCUSSION
In this case series, we demonstrate the efficacy of neoadjuvant lenvatinib therapy to downstage unresectable LATC, ultimately facilitating surgical resection in four out of five cases (80%).
The use of neoadjuvant TKIs in unresectable LATC is showing promise, with significant radiological response, facilitating the preservation of major neurovascular structures [12-37]. In our series, the aims of commencing neoadjuvant therapy varied among patients. In three cases, this was to achieve local disease control, allowing resection without sacrifice of critical vascular structures and avoiding the need for laryngectomy. In two patients, lenvatinib was initiated with palliative intent to treat distant disease, however its therapeutic effect combined with optimisation of patient comorbidities facilitated subsequent surgery.
As the optimal duration of neoadjuvant MKI therapy has not been established and the durability of treatment response remains unknown, our patients were reviewed every three months to assess treatment response. The MDT recommended surgery as soon as the locoregional disease was considered resectable on imaging, to reduce the risk of developing resistance to pathway inhibition and disease progression. In our series, patients received a median of 5.5 months (range 1.6-8.3 months) of neoadjuvant therapy and lenvatinib was typically withheld for a median of 11.5 days (range 8-14 days) before surgery. As TKIs affect the proliferation of endothelial cells and cause adverse effects including HTN, hand-foot skin reactions and thromboembolism. These side effects range in severity and may require dose reduction, interruption or premature cessation of therapy [26-40]. In the SELECT study, across all grades adverse events were reported in 97.3% of patients receiving lenvatinib [24]. Treatment related HTN was the most common treatment related side effect, reported by 60% of patients, followed by oral ulcers (40%) and hand-foot syndrome (20%). This is consistent with previous studies which report a 70% incidence of all grades of HTN [41]. In our series, adverse events were managed with dose reduction and no patients required premature lenvatinib cessation. Although we did not observe increased bleeding during surgery or treatment related fistula formation, clinicians need to consider the risks of impaired wound healing, haemorrhage and tracheoesophageal fistula formation which have been reported with lenvatinib use [42-46].
Previous case series have demonstrated a survival benefit for neoadjuvant therapies targeting BRAF status in ATC and RET status in MTC, however, the efficacy of MKIs in the context of specific genetic driver mutations is less established. Although three patients had specific targetable mutations in our study (two patients had RET-mutated MTC, and one had BRAF V600E mutated PTC), lenvatinib was used instead of targeted therapy due to ease of compassionate access. In Australia, TKIs are typically reserved for patients with metastatic thyroid cancer or progressive disease, with selpercatinib only funded for MTC patients with disease recurrence after surgical management. As the results of several case series and clinical trials become available, increased access to selective RET inhibitors and BRAF / MEK inhibitors may allow refinement of neoadjuvant treatment pathways in Australia [47,48]. Until such time, our experience highlights real-world challenges in timely access to selective TKI and targeted therapies and presents neoadjuvant lenvatinib as a reasonable therapeutic alternative.
Our study is limited to five patients who received neoadjuvant lenvatinib for LATC. Due to this small sample size and heterogeneity of tumour histology in our series, we are unable to define predictive factors for response to lenvatinib treatment. Furthermore, although response to therapy was assessed using RECIST criteria, this does not capture changes in the profile of operative complexity and associated surgical morbidity. Currently there are no validated methodologies that assess change in surgical morbidity, however the MGH/MEEI-MSK-MDAnderson (MMM) and Invasive Thyroid Class score is being assessed in a current phase 2 clinical trial [49]. Additionally, the variability of adjuvant therapy administered prevents further analysis of long term durability of lenvatinib on locoregional control.
Lenvatinib demonstrates efficacy as neoadjuvant treatment for LATC, enabling safer surgical resection and minimising surgical morbidity. Further studies are needed to explore the ideal dose, duration and cessation period of neoadjuvant MKI therapies to optimise disease-free progression and overall survival.
CONCLUSION
Neoadjuvant multikinase inhibitor therapy demonstrates efficacy to facilitate surgery in LATC. The management approach and timing of surgery should be tailored to the individual patient’s anatomy, tumour characteristics and therapeutic intent to enhance surgical outcomes and improve overall survival.
REFERENCES
- Welfare AIoHa. Cancer data in Australia. 2024.
- Wang LY, Nixon IJ, Patel SG, Palmer FL, Tuttle RM, Shaha A, et al. Operative management of locally advanced, differentiated thyroid cancer. Surgery. 2016; 160: 738-746.
- Nixon IJ, Simo R, Newbold K, Rinaldo A, Suarez C, Kowalski LP, et al. Management of Invasive Differentiated Thyroid Cancer. Thyroid. 2016; 26: 1156-1166.
- Segal K, Shpitzer T, Hazan A, Bachar G, Marshak G, Popovtzer A. Invasive well-differentiated thyroid carcinoma: effect of treatment modalities on outcome. Otolaryngol Head Neck Surg. 2006; 134: 819- 822.
- Holoubek SA, Yan H, Khokar AH, Kuchta KM, Winchester DJ, Prinz RA, et al. Aggressive variants of papillary thyroid microcarcinoma are associated with high-risk features, but not decreased survival. Surgery. 2020; 167: 19-27.
- Jannin A, Escande A, Al Ghuzlan A, Blanchard P, Hartl D, Chevalier B, et al. Anaplastic Thyroid Carcinoma: An Update. Cancers (Basel). 2022; 14: 1061.
- Shindo ML, Caruana SM, Kandil E, McCaffrey JC, Orloff LA, Porterfield JR, et al. Management of invasive well-differentiated thyroid cancer: an American Head and Neck Society consensus statement. AHNS consensus statement. Head Neck. 2014; 36: 1379-1390.
- Ibrahimpasic T, Ghossein R, Carlson DL, Chernichenko N, Nixon I, Palmer FL, et al. Poorly differentiated thyroid carcinoma presenting with gross extrathyroidal extension: 1986-2009 Memorial Sloan- Kettering Cancer Center experience. Thyroid. 2013; 23: 997-1002.
- Park H, Park J, Park SY, Kim TH, Kim SW, Chung JH. Clinical Course from Diagnosis to Death in Patients with Well-Differentiated Thyroid Cancer. Cancers (Basel). 2020; 12: 2323.
- Babu G, Ravikumar R, Rafi M, Nair LK, Sharafuddin Z, Mathew J, et al. Systemic Therapy in Thyroid Cancer. 2023. In: Thyroid Cancer - The Road from Genes to Successful Treatment.
- Sarvestani AL, Lambdin J, Hu M, Cabanillas M, Waguespack SG, Hernandez JM, et al. Selpercatinib Before Surgery for the Treatment of RET-Altered Thyroid Cancers. Ann Surg Oncol. 2024; 31: 2202- 2203.
- Cleary JM, Sadow PM, Randolph GW, Palmer EL, Lynch TP, Nikiforov YE, et al. Neoadjuvant treatment of unresectable medullary thyroid cancer with sunitinib. J Clin Oncol. 2010; 28: e390-e392.
- Huang NS, Wei WJ, Xiang J, Chen JY, Guan Q, Lu ZW, et al. The Efficacy and Safety of Anlotinib in Neoadjuvant Treatment of Locally Advanced Thyroid Cancer: A Single-Arm Phase II Clinical Trial. Thyroid. 2021; 31: 1808-1813.
- Grasic Kuhar C, Lozar T, Besic N, Music Marolt M. Outcome of Patients with Locally Advanced Metastatic Medullary Thyroid Cancer and Induction Therapy with Tyrosine Kinase Inhibitors in Slovenia. Adv Ther. 2021; 38: 5684-5699.
- Farlow JL, McCrary HC, Sipos JA, Phay JE, Konda B, Agrawal A. Neoadjuvant dabrafenib and trametinib for functional organ preservation in recurrent BRAF V600E-mutated papillary thyroid cancer. Oral Oncol. 2023; 147: 106625.
- Maniakas A, Dadu R, Busaidy NL, Wang JR, Ferrarotto R, Lu C, et al. Evaluation of Overall Survival in Patients With Anaplastic Thyroid Carcinoma, 2000-2019. JAMA Oncol. 2020; 6: 1397-1404.
- Cabanillas ME, Habra MA. Lenvatinib: Role in thyroid cancer and other solid tumors. Cancer Treat Rev. 2016; 42: 47-55.
- Capozzi M, De Divitiis C, Ottaiano A, von Arx C, Scala S, Tatangelo F, et al. Lenvatinib, a molecule with versatile application: from preclinical evidence to future development in anti-cancer treatment. Cancer Manag Res. 2019; 11: 3847-3860.
- Scott LJ. Lenvatinib: first global approval. Drugs. 2015; 75: 553-560.
- Capp C, Wajner SM, Siqueira DR, Brasil BA, Meurer L, Maia AL. Increased expression of vascular endothelial growth factor and its receptors, VEGFR-1 and VEGFR-2, in medullary thyroid carcinoma. Thyroid. 2010; 20: 863-871.
- Khalid H, Sattar F, Ahmad I, Junior VFP, Nishan U, Ullah R, et al. Computer-assisted discovery of natural inhibitors for platelet- derived growth factor alpha as novel therapeutics for thyroid cancer. Front Pharmacol. 2025; 15: 1512864.
- Fallahi P, Ferrari SM, Galdiero MR, Varricchi G, Elia G, Ragusa F, et al. Molecular targets of tyrosine kinase inhibitors in thyroid cancer. Semin Cancer Biol. 2022; 79: 180-196.
- Perez CA, Arango BA, Velez M, Raez LE, Santos E. Emerging role of multikinase inhibitors for refractory thyroid cancer. Biologics. 2012; 6: 257-265.
- Schlumberger M, Tahara M, Wirth LJ, Robinson B, Brose MS, Elisei R, et al. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N Engl J Med. 2015; 372: 621-630.
- Tahara M, Kiyota N, Yamazaki T, Chayahara N, Nakano K, Inagaki L, et al. Lenvatinib for Anaplastic Thyroid Cancer. Front Oncol. 2017; 7: 25.
- Takahashi S, Kiyota N, Yamazaki T, Chayahara N, Nakano K, Inagaki L, et al. A Phase II study of the safety and efficacy of lenvatinib in patients with advanced thyroid cancer. Future Oncol. 2019; 15: 717-726.
- Tsuboi M, Takizawa H, Aoyama M, Tangoku A. Surgical treatment of locally advanced papillary thyroid carcinoma after response to lenvatinib: A case report. Int J Surg Case Rep. 2017; 41: 89-92.
- Schwartz LH, Litière S, de Vries E, Ford R, Gwyther S, Mandrekar S, et al. RECIST 1.1-Update and clarification: From the RECIST committee. Eur J Cancer. 2016; 62: 132-137.
- Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009; 45: 228-247.
- Russell M, Gild ML, Wirth LJ, Robinson B, Karcioglu AS, Iwata A, et al. Neoadjuvant therapy to improve resectability of advanced thyroid cancer: A real-world experience. Head Neck. 2024; 46: 2496-2507.
- Danilovic DLS, Castro G Jr, Roitberg FSR, Vanderlei FAB, Bonani FA, Freitas RMC, et al. Potential role of sorafenib as neoadjuvant therapy in unresectable papillary thyroid cancer. Arch Endocrinol Metab. 2018; 62: 370-375.
- Milner TD, Ronghe M, Shaikh MG, MacGregor FB, Reed N. Vandetanib Tumor Shrinkage in Metastatic Medullary Thyroid Cancer Allowing Surgical Resection of the Primary Site: A Case Report. J Pediatr Hematol Oncol. 2019; 41: e329-e332.
- Damásio I, Simões-Pereira J, Donato S, Horta M, Cavaco BM, Rito M, et al. Entrectinib in the neoadjuvant setting of anaplastic thyroid cancer: a case report. Eur Thyroid J. 2022; 12: e220179.
- Nava CF, Scheffel RS, Cristo AP, Ferreira CV, Weber S, Zanella AB, et al. Neoadjuvant Multikinase Inhibitor in Patients With Locally Advanced Unresectable Thyroid Carcinoma. Front Endocrinol (Lausanne). 2019; 10: 712.
- Stewart KE, Strachan MWJ, Srinivasan D, MacNeill M, Wall L, Nixon IJ. Tyrosine Kinase Inhibitor Therapy in Locally Advanced Differentiated Thyroid Cancer: A Case Report. Eur Thyroid J. 2019; 8: 102-107.
- Zhang Y, Deng X, Ding Z, Kang J, Wu B, Guo B, et al. Preoperative neoadjuvant targeted therapy with apatinib for inoperable differentiated thyroid cancer: A case report. Medicine (Baltimore). 2021; 100: e25191.
- Contrera KJ, Gule-Monroe MK, Hu MI, Cabanillas ME, Busaidy NL, Dadu R, et al. Neoadjuvant Selective RET Inhibitor for Medullary Thyroid Cancer: A Case Series. Thyroid. 2023; 33: 129-132.
- Kim M, Jin M, Jeon MJ, Kim EY, Shin DY, Lim DJ, et al. Lenvatinib Compared with Sorafenib as a First-Line Treatment for Radioactive Iodine-Refractory, Progressive, Differentiated Thyroid Carcinoma: Real-World Outcomes in a Multicenter Retrospective Cohort Study. Thyroid. 2023; 33: 91-99.
- Fleeman N, Houten R, Chaplin M, Beale S, Boland A, Dundar Y, et al. A systematic review of lenvatinib and sorafenib for treating progressive, locally advanced or metastatic, differentiated thyroid cancer after treatment with radioactive iodine. BMC Cancer. 2019; 19: 1209.
- Rendl G, Sipos B, Becherer A, Sorko S, Trummer C, Raderer M, et al. Real-World Data for Lenvatinib in Radioiodine-Refractory Differentiated Thyroid Cancer (RELEVANT): A Retrospective Multicentric Analysis of Clinical Practice in Austria. Int J Endocrinol. 2020; 2020: 8834148.
- Wu H, Ding X, Zhang Y, Li W, Chen J. Incidence and risk of hypertension with lenvatinib in treatment of solid tumors: An updated systematic review and meta-analysis. J Clin Hypertens (Greenwich). 2022; 24: 667-676.
- Koehler VF, Berg E, Adam P, Weber GL, Pfestroff A, Luster M, et al. Real-World Efficacy and Safety of Multi-Tyrosine Kinase Inhibitors in Radioiodine Refractory Thyroid Cancer. Thyroid. 2021; 31: 1531-1541.
- Hamidi S, Boucher A, Lemieux B, Rondeau G, Lebœuf R, Ste-Marie LG, et al. Lenvatinib Therapy for Advanced Thyroid Cancer: Real-Life Data on Safety, Efficacy, and Some Rare Side Effects. J Endocr Soc. 2022; 6: bvac048.
- Kim SY, Kim SM, Chang H, Kim BW, Lee YS, Chang HS, et al. Safetyof Tyrosine Kinase Inhibitors in Patients with Differentiated Thyroid Cancer: Real-World Use of Lenvatinib and Sorafenib in Korea. Front Endocrinol (Lausanne). 2019; 10: 384.
- Locati LD, Piovesan A, Durante C, Bregni M, Castagna MG, Zovato S, et al. Real-world efficacy and safety of lenvatinib: data from a compassionate use in the treatment of radioactive iodine-refractory differentiated thyroid cancer patients in Italy. Eur J Cancer. 2019; 118: 35-40.
- Cabanillas ME, Takahashi S. Managing the adverse events associated with lenvatinib therapy in radioiodine-refractory differentiated thyroid cancer. Semin Oncol. 2019; 46: 57-64.
- Dickerson K, Milas M, Metzger R, Tomeh C, Shellenberger T, Ahmad I, et al. Neoadjuvant systemic therapy for inoperable differentiated thyroid cancers: Impact on tumor resectability. Surgery. 2025; 177: 108836.
- Yeo JJY, Stewart K, Maniam P, Arman S, Srinivasan D, Wall L, et al. Neoadjuvant tyrosine kinase inhibitor therapy in locally advanced differentiated thyroid cancer: a single centre case series. J Laryngol Otol. 2023; 137: 1237-1243.
- Ahmed AHA, Russell MD, Kyriazidis N, Karcioglu AS, Sherman EJ, Ho AL, et al. A phase 2 study of neoadjuvant lenvatinib in locally advanced invasive thyroid cancer. American Society of Clinical Oncology: J Clin Oncol. 2023.