Reboot Surgery: From Concept to Technique and Results
- 1. Department of Otorhinolaryngology Head and Neck Surgery, Cliniques de L’Europe, Europe Hospitals, Brussels, Belgium
- 2. Department of Otorhinolaryngology, Ghent University, Belgium
Abstract
Recent Findings: Despite endoscopic sinus surgery (ESS) being the standard treatment for Chronic rhinosinusitis with nasal polyps (CRSwNP), a high recurrence rate and remaining olfactory complaints often characterize the postoperative period in eosinophilic nasal polyp disease. Reboot surgery was proposed for patients with recurrent and severe CRSwNP, evtl. complicated by asthma comorbidity, to answer to these shortcomings.
Purpose of Review: The purpose of this review was to summarize the current literature on the indication of non-mucosa sparing Reboot surgery for patients with eosinophilic (Type-2) severe uncontrolled Chronic rhinosinusitis with nasal polyps (eCRSwNP), especially when former surgery failed, providing long-term sense of smell and polyp-free status.
Summary: The extent of the surgery in CRS generally depends on the extension and severity of the disease, but also should be guided by the type of sinus inflammation. Reboot surgery has been developed to remove the mucosal memory of triggers leading to the modulation of healthy mucosa into Type-2 eosinophilic disease and allow the growth of a functional respiratory epithelium covering the sinuses. Questions about the microstructure and function of the mucosa after reboot surgery remained unsolved initially; however, long-term follow-up studies of patients with electron microscopical evaluations answer these questions today and are summarized in this review. Further, we aimed to describe the current understanding of indications for Reboot surgery and post-operative long-term results.
Keywords
• Chronic Rhinosinusitis; Endoscopic Sinus Surgery; Nasal Polyps; Olfaction Disorders; Reboot Surgery.
Citation
Gomes SC, Bachert C (2025) Reboot Surgery: From Concept to Technique and Results. Ann Otolaryngol Rhinol 12(4): 1364.
ABBREVIATIONS
AIT: Allergy Chronic Rhinosinusitis; CRSsNP: Chronic Rhinosinusitis without Nasal Polyposis; CRSwNP: Chronic Rhinosinusitis with Nasal Polyposis; CT: Computed Tomography; DREA: Respiratory disease exacerbated by Non-steroidal Anti-inflammatory; ECP: Eosinophilic Catholic Protein; EESS: Extensive Endoscopic Sinus Surgery; EPOS: European Position Paper on Rhinosinusitis and Nasal Polyp; FESS: Functional Endoscopic Sinus Surgery; GCS: Glucocorticosteroids; HRQoL: Health-related quality of lif; IgE: Imunoglobuline-E; I: Interleukine; INCS: Intranasalcorticosteroids; LoS: Losofsmell Score; mAbs: Monoclonal Antibodies; NCS: Nasal Congestion Score; NP: Nasal Polyp; NPS: Nasal Polyp Score; SIT: Specific Immunotherapy; SNOT-22: Synonasal Outcome Test – 22 (Sinonasal result test - 22); Th2: THelper 2 (T auxiliary 2); VAS: Visual Analogue Scale.
INTRODUCTION
Chronic rhinosinusitis with nasal polyposis (CRSwNP), is characterized by Type 2 eosinophilic mucosal inflammation in about 85% of the patients [1-5], depending on the geographic region and ethnicity of the patient. Nasal polyps tend to recur, especially in Type-2 cases and in patients with asthma comorbidity. The recurrence rate in Type 2 patients in Europe and in the United States reaches >90% [6,7]. Nowadays, the understanding of mucosal inflammation has developed, and it became clear that in Type 2 disease not a single cell is in a healthy condition, but all cells are severely transformed, making broad changes in immune response and remodeling mechanisms in CRSwNP [8]. In other words, eosinophils are only an indicator of the Type 2 inflammation. In the cases of uncontrolled severe patients, the normal mucosal defense is inhibited due to differences in the cellular compositions, transcriptomes, proteomes, and deviations in the immune profiles of T cell and B cell receptors, as well as alterations in the intercellular communications [8-10]. The treatment with monoclonal antibodies (mAbs) for these cases has been shown effective with Dupilumab, which treats nasal polyps (NP) growth and inflammation and restores Type 1 immunity [11-13].Another approach may be surgery, however, conventional Functional endoscopic sinus surgery (FESS), especially minimal invasive, is not suitable for bilateral CRSwNP. Originally, it has been developed for ostiomeatal complex diseases and Chronic rhinosinusitis without nasal polyposis (CRSsNP) [14]. In CRSwNP cases, this approach of “restoration of ventilation and drainage” only allowed local medications to better reach the sinuses and does not solve the primary mucosal problem [15,16]. Therefore, the surgical technique of Reboot surgery came to better solve these severe uncontrolled CRSwNP cases [17,18]. The indications for Reboot surgery today are severe Type 2 mucosal disease [15], and mAbs not available or not registered in a country, or not affordable/not covered by the health insurance. In cases that the biological treatment is available, patients must decide for surgery or biologics, after appropriate information was provided for both solutions by the doctor [1-12]. The aim of Reboot approach is a disease-modifying effect, showed by a long term effect on nasal disease and its consequences (better quality of life scores - HRQoL, SNOT-22, LoS, VAS, NCS - better performance in profession life, etc.), and possibly also on lower airways [16-23]. Reboot surgery improved and maintained olfactory function and significantly suppressed NP disease recurrence for at least 2 years [17 24]. Therefore, if there are no contraindications for surgery based on a high surgical risk, Reboot should be considered.
Appropriate choice of the surgical technique: phenotyping, endotyping and selective indication
The Reboot technique should be considered for patients with severe and uncontrolled chronic rhinosinusitis with nasal polyposis (CRSwNP), especially when previous classical endoscopic sinus surgery (ESS) has failed to maintain adequate olfactory function and polyp-free status. However, as a first-line approach, it is indicated for patients at higher risk of polyp recurrence, especially those with associated asthma. Therefore, it is important to highlight the need of a complete preoperative diagnosis of chronic rhinosinusitis (CRS), aiming for a better therapeutic indication according to the type and severity of the disease [18-24]. Severe CRSwNP is defined as bilateral, with a nasal polyp score (NPS) of at least 4 out of 8 points, and persistent symptoms, requiring treatment in addition to the first-line topical treatment intranasal corticosteroids (INCS) and, eventually, to a short course of oral glucocorticosteroids (GCS). Uncontrolled CRSwNP is defined as persistent or recurrent disease, despite long-term treatment with INCS and having received at least 1 course of systemic GCS in the previous 2 years (or having a medical contraindication or intolerance to systemic corticosteroid) and/or previous sinonasal surgery (unless there is a medical contraindication or the patient does not wish to undergo surgery) [1]. By endotyping, it was demonstrated in large studies that CRSwNP is part of the Type-2 inflammatory spectrum of CRS. More specifically, most cases with a severe and uncontrolled phenotype were defined as endotype CRSwNP Type-2, an airway disease mainly orchestrated by Type-2 inflammatory cytokines. Type-2 occurs frequently and, besides by the eosinophilia in the blood, it can be also diagnosed by high levels of eosinophils in histopathological analysis from biopsies, if no GCS was used as recent treatment [1-27]. In a simpler definition, the Type-2 inflammation in the respiratory mucosa is based on the expression of the cytokines IL-4, IL-5, IL 13 and related cells, such as mast cells, eosinophils and Th2 lymphocytes [2-28]. Lately, multi-omic scRNA-seq with bulk RNAseq revealed broad changes in immune response and remodeling mechanisms in CRSwNP. It has been demonstrated that broad expansions occur of CD4 effector/tissue-resident memory T cells, CD8 T effector memory cells and B cells in nasal polyp. The T and B cell receptor repertoires were also skewed in NP [8]. In daily clinical practice, an algorism has been proposed based on the presence of associated asthma and/or the number of eosinophils in the peripheral blood greater than 300/µl [1-29].Maintaining a polyp-free status, without the need for additional surgical intervention, and preserving olfactory and gustatory functions, are the main demands of the patients with uncontrolled severe CRSwNP. Thus, Reboot surgery reinforces the concept of disease-modifying effect, specifically for CRSwNP, which will be further detailed.
The mucosal concept
In recent years, there has been a great evolution in understanding the pathophysiological mechanisms of CRS, from phenotyping to endotyping, from eosinophilic inflammation to Type-2 immunity [1-30]. Recently, the evolution of the standard “drainage and ventilation” concept of the sinuses to the “mucosal concept” has been established, placing mucosal inflammation at the center of our understanding [15-23]. It is demonstrable that CRS endotypes are more complex than previously assumed, with heterogeneity of characteristics, especially in the subgroup with nasal polyposis [2-5]. Thanks to the advent of endotyping and biomarker screening, therapeutic options have become more precisely tailored to inflammation and more individualized [31], with clinical and surgical consequences [7-36].The mucosal concept is based on the knowledge acquired through the progress in understanding patho immunology. The sinus mucosa in patients with CRS is a reservoir of inflammatory cells and markers. Therefore, cases with a high local inflammatory burden, such as patients with severe and uncontrolled CRSwNP, require more personalized treatments, such as the use of biologics and/or extended surgeries with a mucosal approach, which go beyond the classic “ventilation and drainage” technique [12-38]. Type-2 inflammation is associated with germs in the intramucosal layer and significant immune dysfunction. The complete removal of the sinus mucosa, along with the local microbiota, have a major impact on the natural course of the disease. Type-2 cytokines, such as IL-4 and IL-13, impair epithelial tight junction organization and barrier formation, support immunoglobulin IgE synthesis by B cells and plasma cells, remodel the vasculature to be more permeable, and impair the development of macrophages [8-40]. Therefore, only complete suppression of Type 2 inflammation and mucosal memory cell populations allows for a normal postoperative course [17-24]. Regardless of the therapeutic choice, the current rhinologist’s approach to managing CRS cases must be based on the rules of precision medicine [12-15]. For the severe uncontrolled Type-2 cases, the Reboot surgery aims at the removal of the entire inflamed sinus mucosa, along with the memory B-cells, T-cells and local microbiota [17,18].
Overview of tailored therapies
In 2019 the term “Reboot surgery” was published for the first time as an approach that, first, removes all inflamed sinus mucosa and, second, hereby allows healthy and undisturbed re-epithelialization. The technique aims to remove the diseased sinus mucosa from all the sinuses. Where possible, the periosteum should be preserved, so that the nasal mucosal epithelium can fast grow from the nasal cavity to the sinuses and cover the bony surfaces with a thin new mucosa [17]. The new layer of sinus mucosa, at 24-month post-Reboot, shows notable reduction in eosinophilic infiltration, a complete regeneration of the ciliated, pseudostratified respiratory epithelium, with intercellular junctions (tight junctions, adherens junctions, and desmosomes), mucus-producing goblet cells, and an underlying lamina propria, that altogether resemble the aspect of normal mucosa. This ensures the normal production and transport of nasal fluids, which directly influences the improvement of clinical symptoms, the SNOT-22 and VAS global scores [23].The Reboot approach shows some similarities with other techniques, as the Centripetal approach from Brazil [34], and the Nasalization approach from France [41]. In practice, they differ in both technique and justification. In 1995 in France, Jankowski and collaborators reinforced the idea of radical ethmoidectomy for recalcitrant CRSwNP. The Nasalization approach, originally in 2003, was based on bilateral surgery with radical endoscopic ethmoidectomy with mucosal resection of the lateral walls of the ethmoids, resection of the middle turbinate, antrostomy, sphenoidotomy and exposure of the frontal ostium. The technique, also classified as Extensive Endoscopic Sinus Surgery (EESS), was successful when compared to the classic endoscopic sinus surgery, due to the decrease in disease recurrence rates [41,42]. The approach evolved in 2018 with new definitions of the Nasalization/EESS and explanations of its main concepts. The group proposed a maximum resection of the ethmoid mucosa. However, although NP from the middle turbinate is very rare [17], they still proposed the resection of the middle conchae to bring the benefit of maximum removal of the so-called vestigial olfactory mucosa [43]. The concept of “ventilation and drainage” of the sinuses was initially challenged by Jankowski’s group, who stated that nasal polyposis would be specific to the olfactory tissue, particularly the vestigial olfactory mucosa of the ethmoid - considered not a sinus, but a bone at the skull base that houses the olfactory mucosa [44]. This justification based only on anatomy has not been supported, as this concept did not include the primary role of the inflammation. On the other hand, in the same year of 2018, the research Belgian group led by Bachert introduced the concept of mucosal disease as the origin of CRS and discussed the immunology and inflammatory markers involved [28]. From then on, this tailored sinonasal surgery was supported, according to nasoendoscopy and computed tomography (CT) findings (phenotype), additionally corroborated by the inflammatory profile (endotype) [45]. The endotype-based diagnosis, after investigation of immune responses and pathological mechanisms, facilitates the prediction of not only the prognosis of the sinus disease, but also the risks of comorbid diseases, such as asthma. The patient guidance became personalized for classical pharmacotherapy and for innovative therapy, introducing the biologics to target cells and Type-2 immune markers, and surgical approaches focusing on the decrease of disease recurrence by removing inflammation. Bachert et al also specialized in the study of biologics to suppress Type-2 inflammation (interleukins IL-4, IL-5 and IL-13; immunoglobulin IgE; eosinophils) by anti-IL5, anti-IgE and anti-IL4Ra, confirming the importance of Type-2 inflammation in the mucosal disease. They and others impressively showed that biologics, specifically Dupilumab, suppressed the mucosal inflammation to an extent that turned surgery often unnecessary. Nowadays, anti-Type-2 biologics are routinely recommended in national and international guidelines and have reduced the use of surgical approaches dramatically. As Reboot surgery is not regularly used by most of the sinus surgeons due to its high technical demands, and biologics are more and more replacing surgery for CRSwNP in many countries [12-49], Reboot surgery should nowadays be reserved for patients in situations where no biologics are available or financially accepted by the patient, and surgeons are experienced to perform Reboot surgery in a safe and successful manner. Lately, new studies were published by several centers specialized in rhinology outside of Ghent or Belgium, confirming the effect of the Reboot technique in short and long term. The authors provided evidence for the improvement of patient’s symptoms and nasal function, including smell and nasal obstruction, and absence of new signs of mucosal edema or recurrence of polyps [16-50]. Such studies increasingly support the indication of Reboot surgery for severe uncontrolled CRSwNP cases, which are treated unsatisfactorily by pharmacological therapies and by repeated revision surgeries using standard endoscopic techniques [18-51].
Reboot technique: step by step Reboot surgery is the technique that aims to completely remove the thickened and inflamed sinus mucosa, including all polyp formation in all affected sinuses, mainly involving the ethmoid and maxillary sinuses bilaterally, as well as part of the mucosa of the sphenoid and frontal sinuses. Furthermore, in case of edema or polyp formation on the middle turbinates, they must be adequately resected. The upper turbinates may also show polyp growth, which must be removed; however, the turbinates should be preserved as much as possible to maintain olfactory function. Additionally, the frontal sinuses may be involved, and a simple resection of the medial part of the frontal mucosa may be necessary, if sufficient space remains to maintain the opening long-term. Otherwise, a resection of the frontal sinus floor anteriorly and laterally may be indicated to maintain access. In cases of difficult access to the frontal sinus and/or extended frontal disease, it may be necessary to combine the Reboot technique with the DRAF III approach (full Reboot), to ensure access to the sinus and removal of the massively affected frontal mucosa.The surgeon must begin the procedure through a wide antrostomy and complete removal of the maxillary sinus mucosa, including the alveolar recess, using 30° and 70° endoscopes (Figures 1, 2 and 3).
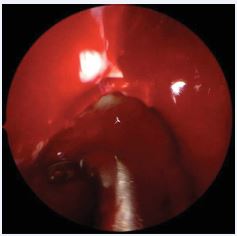
Figure 1 Reboot surgery approach. Use of 360o rotating antrum forceps in the maxillary sinus, right side.
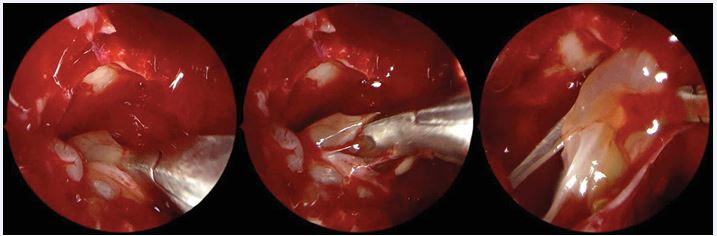
Figure 2 Reboot surgery approach. Image sequence showing the antrum grasping forceps (Heuwieser) removing polypoid mucosa from the entire right maxillary sinus.
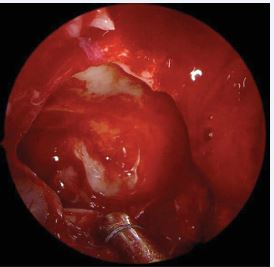
Figure 3 Exposure of the periosteum of the right maxillary sinus after removal of the lining diseased mucosa.
Next, the diseased mucosa of the anterior and posterior ethmoids must be removed, including lamina papyracea, skull base, and lateral face (and anterior portion, when necessary) of the middle turbinate (Figure 4).
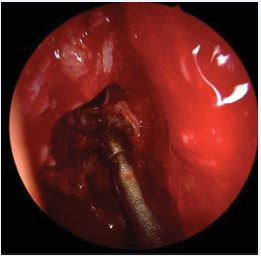
Figure 4 Removal of diseased mucosal tissue from the right ethmoid sinus using blunt, curved, atraumatic suction instruments.
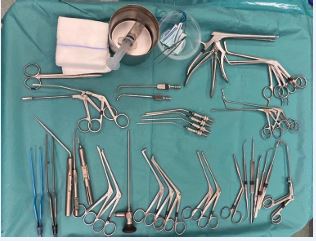
The sphenoid sinus requires specific attention to the main structures that pass along its roof and side walls (internal carotid arteries and optic nerves). Mucosal removal occurs under direct endoscopic visualization through a wide access created by removing the posterior ethmoid and anterior sphenoid wall (up to the base of the skull) and must be restricted to the floor mucosa and medial walls of the sinus. Next, the frontal recess is accessed to completely remove the mucosa from the anterior skull base. The middle turbinate is preserved as an anatomical landmark, except for areas affected by the disease or its anterior portion, which can be removed for a better frontal access. The upper turbinate needs to be partially removed when diseased; this also especially helps with access to the sphenoid sinus and the skull base. Finally, the DRAF III procedure is performed to provide maximum access to both frontal sinuses by reducing the bony walls laterally and anteriorly and removing the inter-frontal septum. Through this wide access, the frontal sinus mucosa is removed from the posterior and anterior walls as laterally as possible. Hemostatic control is then performed, and nasal tampons are placed bilaterally in the middle meatus and nasal cavities, being removed the day after.The intention of surgery is to minimize the inflammatory load present in the mucosa, although complete removal in the frontal, the sphenoid and in the alveolar recesses of the maxillary sinuses may not be completely possible due to limitations in visualization and instrumentation. Table 1 and Figure 5 show the surgical equipment and instruments necessary for a safe and effective performance of Reboot surgery. Additionally, it is essential to use 4 mm 30° and 70° nasal endoscopes, video screen, camera and quality light source in a properly equipped surgical center.
Figure 5 Table of instruments for Reboot endoscopic nasal surgery.
Table 1: Surgical instruments for each sinus in Reboot surgery.
|
SINUS |
INSTRUMENTS |
|
Maxillary Sinus |
70o endoscope + Antrum grasping forceps (Heuwieser) + 90o Bakesley |
|
Ethmoid sinus |
45o up-biting Blakesly + Microdebrider with 60o blade at 5000 rpm rotation speed |
|
Sphenoid sinus |
Hajek-Kofler Sphenoide punch fórceps 360o rotary + Microdebrider (used only on the floor and medial portion) |
|
Frontal sinus DRAF III |
Frontal sinus punch forceps + Frontal sinus high-speed Midas Rex drill (Medtronic) + Frontal sinus seeker + Frontal sinus curette |
After understanding the selection of appropriate patients, the concept of mucosa and the surgical technique itself, it is also important to demystify the main objective of the technique: the disease-modifying effect.
Immunology: the disease-modifying effect
The concept of disease modification explains the achievement of lasting benefits after an intervention. Studies on modifying the natural history of the disease in cases of Type-2 IgE-mediated cases such as allergic rhinitis, asthma, food allergies and atopic dermatitis are already well established [52]. This concept is described in the literature, as it encompasses allergen immunotherapy (AIT), which leads to a profound suppression of the inflammation [53,54]. In the 1960s, the first randomized clinical trials with specific immunotherapies (SIT) were carried out, bringing for the first time the general concept of disease modification. With its evolution, SIT demonstrated additional effects, such as persistence of long-term benefits even after discontinuation of therapy, prevention of new sensitizations and reduction of the risk of asthma onset in children with allergic rhinitis [52].
It is important to highlight that although AIT is an example for a disease-modifying effect, it is not used as a treatment for CRSwNP. However, as nasal polyposis is also a Type-2 inflammation, the hypothesis was raised to extend the disease modification concept to Reboot surgery for CRSwNP. Biological therapy does not seem to induce a change in immunological memory in nasal polyposis, even after several years of treatment, although it is well known for asthma [55-57]. The disease-modifying effect that Reboot surgery achieves is based on the replacement of inflamed sinus mucosa with healthy nasal mucosa, which can lead to local suppression of circulating eosinophils and bone marrow precursors [17,18]. This structural change is reflected in the clinic, as the improvement in signs and symptoms is maintained in the long term. In addition to reducing the recurrence of nasal polyposis, these effects are also demonstrated to provide long-lasting improvement in the sense of smell through olfactory plasticity [24].
From microstructure to clinical practice
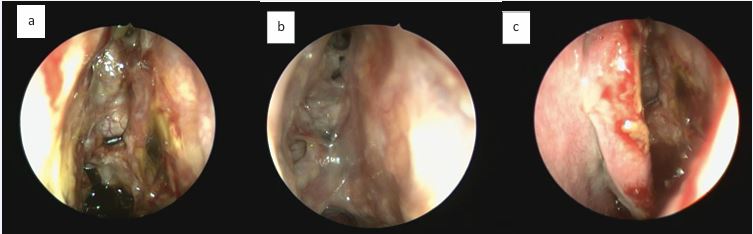
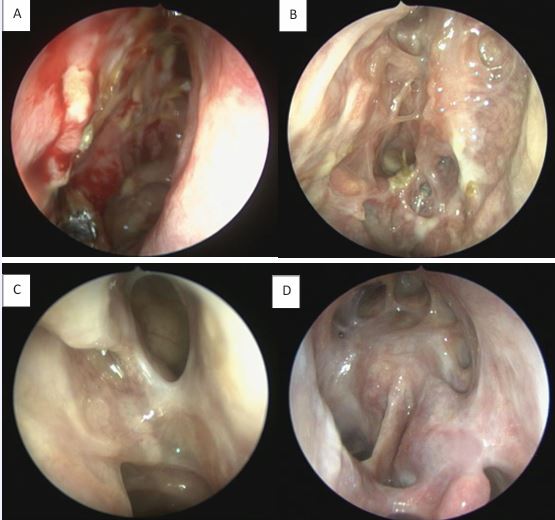
The olfactory mucosa of severe uncontrolled CRSwNP also presents typical markers of Type-2 inflammation (IgE, IL4, IL5, ECP, CCL3 and CCL4), similar to the pattern present in the nasal polyps. Patients post-Reboot demonstrated significant improvement in olfactory function, with significant response at just 1 month post-operatively and continued improvement at 2 years of follow-up. In contrast, ESS surgery showed improvement in olfactory function in the short-term follow-up, but worsened smell in the long term; Reboot also showed lower recurrence rates of nasal polyps when compared to ESS surgery at 2-year follow-up [17-24].Type-2 inflammation in nasal polyps is associated with worse impaired sense of smell before surgery but with a significant better olfactory improvement after Reboot. In other words, the more severe the Type-2 inflammation is in the polyps pre-operatively, the more significant the olfactory improvement is in the Reboot post-operative. There is no significant correlation between smell and Type-2 inflammation in the olfactory mucosa tissue, before or after surgery. Possibly, Type-3 inflammation in the olfactory mucosa is associated with better pre operative smell, but worse olfactory evolution after Reboot [24]. Postoperative nasoendoscopy images show that, by keeping the periosteum as intact as possible during surgery, the sinus mucosa re-epithelializes on average within 2 weeks, following with satisfactory long-term evolution (Figures 6 and 7).
Figure 6 Postoperative period of Reboot surgery at 2 weeks: return of nasal mucosa in the ethmoid sinus in 3 different patients. A) Left nasal cavity, reepithelization process; B) Left fronto-ethmoidal region, visualization of the frontal recess with adequate opening; C) Left nasal cavity, no signs of nasal synechia.
Figure 7 Postoperative follow-up after Reboot surgery. A) Right nasal cavity, 14 days; B) Right nasal cavity, 30 days; C) Left nasal cavity, 90 days; D) Left nasal cavity, DRAF III, 24 months.
In clinical practice, the formation of sinus scars has been rarely observed; on the contrary, the mucosa remains hydrated and functional, without disturbance of mucociliary clearance, measured by the saccharin transport test, and patients do not report a feeling of dryness in the nasal cavity [22,23]. Furthermore, it is well known by otorhinolaryngologists that resection of the sinus mucosa is often necessary in cases of benign or malign tumors of the sinuses, progressing post-operatively with adequate mucosal restructuring [58-60]. We have learned that extensive surgery can lead to increased complication rates and/or more severe complications. However, comparative studies between extensive surgery and minimal approaches have shown that there were no measurable differences between groups regarding the rates or severity of complications [18-61]. But it is important to remember that the Reboot procedure should only be performed by a surgeon experienced in endoscopic sinus surgery, including DRAF III procedures.
DISCUSSION
As mentioned in the most recent European Position Paper on Rhinosinusitis and Nasal Polyps (EPOS), sinus surgery is “functional” when ventilation and drainage are improved, a sinus cavity is created that incorporates the natural ostium, mucociliary clearance is facilitated, and better conditions are provided for local treatment [30]. Therefore, Reboot surgery can be interpreted as “functional” surgery, since healthy re-epithelialization occurs [19]. Reboot surgery, by completely removing the diseased mucosa from the paranasal sinuses, significantly reduces the recurrence of nasal polyps in Type-2 CRSwNP for a longer period, when compared to the current ESS approach with mucosal preservation. Alsharif et al. showed that 45% of patients relapsed within 2 years after the classic ESS approach, 17% relapsed after partial Reboot and only 1 patient (8%) relapsed after Reboot + DRAFIII, with significantly different rates between ESS and Reboot (p=0.02), as well as among all groups (p=0.038)(17). Endotyping the patient is a fundamental key for a personalized treatment choice. Xu et al., by using single-cell RNA sequencing, transcriptomics, surface proteomics, and T and B cell receptor sequencing (multi-omics), identified differences in the cellular compositions and deviations in the immune profiles of both T and B cell receptors, as well as alterations in the intercellular communications in uncontrolled severe CRSwNP patients, which might help to define potential therapeutic targets in the future [8]. Interestingly, the methods for endotyping in clinical practice are becoming simpler. Paoletti and colleagues presented that nasal cytology is a suitable tool for assessing local biomarkers of Type-2 inflammation in CRSwNP [37]. Severe uncontrolled CRSwNP is a long-lasting and disabling disease due to olfactory dysfunction, nasal symptoms associated with obstruction, and a high probability of recurrence. Reboot significantly improved olfactory function, which was maintained long-term for at least 2 years in early retrospective studies [17,18]. Gomes et al., presented by the complete Sniffin’ Sticks test (TDI) a significant improvement in smell within a month after surgery, which was maintained for at least 6 months [24]. Malvezzi et al., observed similar results after Reboot, with sharp and rapid improvement of sinonasal symptoms and a remarkable elongation of time to relapse, compared to previous treatments. This included progressive improvement in the sense of smell and taste in 1, 2 and 4 weeks of follow-up after surgery [22]. Chen et al., showed that in patients with eosinophilic chronic rhinosinusitis occurs tissue eosinophilia-induced apoptosis and turnover disruption of olfactory sensory neurons, leading to olfactory disfunction independently of polyps and disease severity. Therefore, treatment with biologics, corticosteroids, and surgery may recover olfactory function by reducing eosinophilic infiltration in the mucosa [38], which is in line with what Reboot surgery offers. Pirola and collaborators confirmed that demucosization, also called by the group as Non-Mucosa Sparing Endoscopic Sinus Surgery (Partial Reboot), allows restoration of infiltrate-free epithelium, and may influence immunopathogenic mechanisms underlying refractory CRSwNP. This reflects with significance on several quality of-life scores [21]. At histological level, re-epithelisation after 10 months was demonstrated by biopsy and histopathological analysis at the level of the maxillary antrostomy, with healthy mucosa that appeared pseudostratified as normal nasal epithelium, with no evidence of relevant eosinophilic infiltration [22]. Further studies on this subject also showed by means of electron microscopy (both in transmission and scanning mode) that, after 24 months from Reboot surgery, the ultrastructure of the sinus mucosa changed significantly, improving the mucosal morphology, collagen composition, vascularity, and cell adhesion, with restoration of the normal epithelium and the ciliary structure and function [23]. With these findings, it is stablished that Reboot sinus surgery is an effective solution for patients with recalcitrant CRSwNP, especially when unresponsive to monoclonal antibodies (mAbs) and that already underwent multiple sinus surgeries [20]. Blauwblomme et al., presented in 2025 an expert concensus on surgical management of Primary Diffuse Type 2-Dominant CRS. The multicontinental group defended that the key focus is a complete sinus surgery, which involves adequate primary and revision surgery, including clearing all nasal polyps and diseased mucosa while ensuring ideal conditions for topical therapy. Moreover, there was also consensus on performing complete sinus surgery before considering monoclonal antibody therapies, unless contraindicated [51]. Nonetheless, several suggestions have been made to quantify the extent of surgery or to standardize the description of surgical interventions. A variety of extended endoscopic procedures can be used in the management of primary diffuse bilateral type 2 chronic rhinosinusitis: Neo-sinus ESS/Full FESS, Nasalization, Nasalization updated, Mucoplasty and Reboot. In the end, the extent of surgery in CRS depends potentially on the severity of the disease and the type of underlying inflammation [19]. In the Full Reboot cases, a flap of healthy mucosa over the periosteum (mucoplasty) was proposed to maintain the mucosal lining and frontal access [35,36]. however, the key focus is the space created for the frontal sinuses rather than the restoration of the sinus mucosa. Eloy and Musat summarized the clinician’s point of view for a better treatment of nasal polyposis. A balanced combination of topical and oral corticosteroids, antibiotics, biologics, and targeted surgery is the best way to provide adequate and lasting control of chronic sinusitis. Surgical options are the nasalization, Draf procedure, or the reboot procedure with complete resection of the mucosa of all the paranasal sinus cavities, in case of major symptomatic recurrences nonrespondent to current treatments [31]. And although these types of extended surgeries are more aggressive, the health-related quality of life after surgery is still preserved [60]. In short, the recurrence of nasal polyps after sinus surgery, at least in part, is a matter of failure to remove the inflammatory burden affecting the various levels of the diseased sinus mucosa. Performing a complete Reboot surgery after a previous partial attempt leads to a significant additional increase in disease-free time. Unlike the sinus mucosa, the nasal mucosa is rarely involved in the formation of polyps. Thus, only complete inflammatory suppression can allow adequate healing, allowing cell migration from the nasal cavity to the sinuses.
LIMITATIONS
Multicenter studies with possible combined approaches would help to establish or to correct the criteria for disease-modifying effects, and to improve the selection of patients who benefit from the procedure. More extensive studies are still needed to better evaluate histologically and functionally the sinus mucosa tissues after Reboot.
CONCLUSION
Although these patients suffer from intense Type-2 inflammation, a significant reduction in disease recurrence occurs after Reboot, with a consequent decrease in the severity and frequency of symptoms. A balanced combination of topical corticosteroids, biologics, and targeted surgery is the best way to provide adequate and lasting control of chronic sinusitis in severe uncontrolled cases. The Reboot technique should be considered for patients with severe, uncontrolled CRSwNP, especially when ESS fails to provide polyp-free status and adequate olfactory function in a short and long term.
ACKNOWLEDGEMENTS
Thanks to the staff members, the residents and the patients from the University Hospital of Ghent (UZ Gent), the University Hospital of Brussels (UZ Brussel), and the University Hospital of São Paulo (Hospital das Clínicas FMUSP) for their support, friendliness and contribution.
REFERENCES
- Bachert C, Han JK, Wagenmann M, Hosemann W, Lee SE, Backer V, et al. EUFOREA expert board meeting on uncontrolled severe chronic rhinosinusitis with nasal polyps (CRSwNP) and biologics: Definitions and management. J Allergy Clin Immunol. 2021; 147: 29-36.
- Tomassen P, Vandeplas G, Van Zele T, Cardell LO, Arebro J, Olze H, et al. Inflammatory endotypes of chronic rhinosinusitis based on cluster analysis of biomarkers. J Allergy Clin Immunol. 2016; 137: 1449-1456.e4.
- Wang X, Zhang N, Bo M, Holtappels G, Zheng M, Lou H, et al. Diversity of TH cytokine profiles in patients with chronic rhinosinusitis: A multicenter study in Europe, Asia, and Oceania. J Allergy Clin Immunol. 2016; 138: 1344-1353.
- Zhang N, Van Zele T, Perez-Novo C, Van Bruaene N, Holtappels G, DeRuyck N, et al. Different types of T-effector cells orchestrate mucosal inflammation in chronic sinus disease. J Allergy Clin Immunol. 2008; 122: 961-968.
- Romano FR, Valera FCP, Fornazieri MA, Lopes NMD, Miyake MM, Dolci RLL, et al. Inflammatory Profile of Chronic Rhinosinusitis With Nasal Polyp Patients in Brazil: Multicenter Study. Otolaryngol Head Neck Surg. 2024; 171: 1552-1561.
- Bachert C, Zhang L, Gevaert P. Current and future treatment options for adult chronic rhinosinusitis: Focus on nasal polyposis. J Allergy Clin Immunol. 2015; 136: 1431-1440.
- Bachert C, Zhang N, Cavaliere C, Weiping W, Gevaert E, Krysko O. Biologics for chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2020; 145: 725-739.
- Xu Z, Huang Y, Meese T, Van Nevel S, Holtappels G, Vanhee S, et al. The multi-omics single-cell landscape of sinus mucosa in uncontrolled severe chronic rhinosinusitis with nasal polyps. Clin Immunol. 2023; 256: 109791.
- Lan F, Zhong H, Zhang N, Johnston SL, Wen W, Papadopoulos N, et al. IFN-λ1 enhances Staphylococcus aureus clearance in healthy nasal mucosa but not in nasal polyps. J Allergy Clin Immunol. 2019; 143: 1416-1425.e4.
- Geng B, Bachert C, Busse WW, Gevaert P, Lee SE, Niederman MS, et al. Respiratory Infections and Anti-Infective Medication Use From Phase 3 Dupilumab Respiratory Studies. J Allergy Clin Immunol Pract. 2022; 10: 732-741.
- Loperfido A, Ciofalo A, Cavaliere C, Begvarfaj E, Cascone F, Alfonzo G, et al. Dupilumab’s Impact on Blood Parameters in Nasal Polyposis: 18-Month Follow-Up in Real Life. J Immunol Res. 2023; 15: 2023.
- Huang Y, Zhang N, Bachert C. Innovative treatments for severe uncontrolled chronic rhinosinusitis with nasal polyps. Expert Rev Clin Immunol. 2023; 19: 837-845.
- Bachert C, Corren J, Lee SE, Zhang H, Harel S, Cunoosamy D, et al. Dupilumab efficacy and biomarkers in chronic rhinosinusitis with nasal polyps: Association between dupilumab treatment effect on nasal polyp score and biomarkers of type 2 inflammation in patients with chronic rhinosinusitis with nasal polyps in the phase 3 SINUS-24 and SINUS-52 trials. Int Forum Allergy Rhinol. 2022; 12: 1191-1195.
- Stammberger H, Posawetz W. Functional endoscopic sinus surgery. Concept, indications and results of the Messerklinger technique. Eur Arch Otorhinolaryngol. 1990; 247: 63-76.
- Huang Y, Zhang N, Xu Z, Zhang L, Bachert C. The Development of the Mucosal Concept in Chronic Rhinosinusitis and Its Clinical Implications. J Allergy Clin Immunol Pract. 2022; 10: 707-715.
- Gomes SC, Delemarre T, Holtappels G, Van Zele T, Derycke L, Bonne E, et al. Olfaction in nasal polyp patients after Reboot surgery: an endotype-based prospective study. Eur Arch Otorhinolaryngol. 2023; 280: 2821-2830.
- Alsharif S, Jonstam K, van Zele T, Gevaert P, Holtappels G, Bachert C. Endoscopic Sinus Surgery for Type-2 CRS wNP: An Endotype-Based Retrospective Study. Laryngoscope. 2019; 129: 1286-1292.
- Gomes SC, Cavaliere C, Masieri S, Van Zele T, Gevaert P, Holtappels G, et al. Reboot surgery for chronic rhinosinusitis with nasal polyposis: recurrence and smell kinetics. Eur Arch Otorhinolaryngol. 2022; 279: 5691-5699.
- Blauwblomme M, Gevaert P, Van Zele T. Chronic Rhinosinusitis: Matching the Extent of Surgery with Pathology or Does the Extent of Surgery Matter? Current Otorhinolaryngology Reports. 2023; 11: 273-285.
- Pirola F, Giombi F, Pace GM, Cerasuolo M, Zuppardo J, Sebastiani M, et al. Non-Mucosa Sparing (Reboot) Surgery as a Possible Rescue Therapy in Patients Locally Unresponsive to Biologics. Ear Nose Throat J. 2024: 1455613241282566.
- Pirola F, Pace GM, Giombi F, Heffler E, Paoletti G, Nappi E, et al. Outcomes of Non-Mucosa Sparing Endoscopic Sinus Surgery (Partial Reboot) in Refractory Chronic Rhinosinusitis with Nasal Polyposis: An Academic Hospital Experience. Laryngoscope. 2023; 133: 1584-1589.
- Malvezzi L, Pirola F, De Virgilio A, Heffler E. Long-lasting clinical, radiological and immunological remission of severe nasal polyposis by means of ‘reboot’ surgery. BMJ Case Rep. 2020; 13: e233726.
- Pirola F, Vezzoli E, Falqui A, Giombi F, Heffler E, Mercante G, et al. Nasal Mucosa Regeneration After Reboot Surgery: Electron Microscopy and Histology Insights in CRSwNP. Laryngoscope. 2025; 135: 3082-3092.
- Gomes SC, Delemarre T, Holtappels G, Van Zele T, Derycke L, Bonne E, et al. Olfaction in nasal polyp patients after Reboot surgery: an endotype-based prospective study. Eur Arch Otorhinolaryngol. 2023; 280: 2821-2830.
- Bachert C, Marple B, Hosemann W, Cavaliere C, Wen W, Zhang N. Endotypes of Chronic Rhinosinusitis with Nasal Polyps: Pathology and Possible Therapeutic Implications. J Allergy Clin Immunol Pract. 2020; 8: 1514-1519.
- Staudacher AG, Peters AT, Kato A, Stevens WW. Use of endotypes, phenotypes, and inflammatory markers to guide treatment decisions in chronic rhinosinusitis. Ann Allergy Asthma Immunol. 2020; 124: 318-325.
- Kato A, Peters AT, Stevens WW, Schleimer RP, Tan BK, Kern RC. Endotypes of chronic rhinosinusitis: Relationships to disease phenotypes, pathogenesis, clinical findings, and treatment approaches. Allergy. 2022; 77: 812-826.
- Bachert C, Zhang N, Hellings PW, Bousquet J. Endotype-driven care pathways in patients with chronic rhinosinusitis. J Allergy Clin Immunol. 2018; 141: 1543-1551.
- hen FH, Deng J, Hong HY, Xu R, Guo JB, Hou WJ, et al. Extensive versus functional endoscopic sinus surgery for chronic rhinosinusitis with nasal polyps and asthma: A 1-year study. Am J Rhinol Allergy. 2016; 30: 143-148.
- Fokkens WJ, Lund VJ, Hopkins C, Hellings PW, Kern R, Reitsma S, et al. European Position Paper on Rhinosinusitis and Nasal Polyps 2020. Rhinology. 2020; 58: 461-464.
- Eloy P, Musat GC. What We Know about Nasal Polyposis: The Clinician’s Point of View. Sinusitis. 2024; 8: 37-50.
- 32. Xu Z, Huang Y, Delemarre T, Cavaliere C, Zhang N, Bachert C. Advances in chronic rhinosinusitis in 2020 and 2021. J Allergy Clin Immunol. 2022; 149: 854-866.
- Wautlet A, Bachert C, Desrosiers M, Hellings PW, Peters AT. The Management of Chronic Rhinosinusitis With Nasal Polyps (CRSwNP) With Biologics. J Allergy Clin Immunol Pract. 2023; 11: 2642-2651.
- Felippu A. Nasal centripetal endoscopic sinus surgery. Ann Otol Rhinol Laryngol. 2011; 120: 581-585.
- Martin-Jimenez D, Moreno-Luna R, Cuvillo A, Gonzalez-Garcia J, Maza- Solano J, Sanchez-Gomez S. Endoscopic Extended Sinus Surgery for Patients with Severe Chronic Rhinosinusitis with Nasal Polyps, the Choice of Mucoplasty: A Systematic Review. Curr Allergy Asthma Rep. 2023; 23: 733-746.
- Moreno-Luna R, Gonzalez-Garcia J, Maza-Solano JM, Molina-Fernandez E, Pinheiro-Neto CD, Del Cuvillo Bernal A, et al. Free nasal floor mucosal grafting after endoscopic total ethmoidectomy for severe nasal polyposis: a pilot study. Rhinology. 2019; 57: 219-224.
- Paoletti G, Malvezzi L, Riccio AM, Descalzi D, Pirola F, Russo E, et al. Nasal cytology as a reliable non-invasive procedure to phenotype patients with type 2 chronic rhinosinusitis with nasal polyps. World Allergy Organ J. 2022; 15: 100700.
- Chen Y, Li M, Lu J. Apoptosis and turnover disruption of olfactory sensory neurons in eosinophilic chronic rhinosinusitis. Front Cell Neurosci. 2024; 18: 1371587.
- Kolkhir P, Akdis CA, Akdis M, Bachert C, Bieber T, Canonica GW, et al. Type 2 chronic inflammatory diseases: targets, therapies and unmet needs. Nat Rev Drug Discov. 2023; 22: 743-767.
- Huang Y, Xu Z, Holtappels G, Shen Y, Van Zele T, Wen W, et al. MZB1- expressing cells are essential for local immunoglobulin production in chronic rhinosinusitis with nasal polyps. Ann Allergy Asthma Immunol. 2024; 132: 198-207.e14.
- Jankowski R, Bodino C. Evolution of symptoms associated to nasal polyposis following oral steroid treatment and nasalization of the ethmoid--radical ethmoidectomy is functional surgery for NPS. Rhinology. 2003; 41: 211-219.
- Jankowski R, Pigret D, Decroocq F, Blum A, Gillet P. Comparison of radical (nasalisation) and functional ethmoidectomy in patients with severe sinonasal polyposis. A retrospective study. Rev Laryngol Otol Rhinol (Bord). 2006; 127: 131-140.
- Jankowski R, Rumeau C, Nguyen DT, Gallet P. Updating nasalisation: From concept to technique and results. Eur Ann Otorhinolaryngol Head Neck Dis. 2018; 135: 327-334.
- ankowski R, Rumeau C, Gallet P, Nguyen DT. Nasal polyposis (or chronic olfactory rhinitis). Eur Ann Otorhinolaryngol Head Neck Dis. 2018; 135: 191-196.
- Aguiar C, Valente P, Medeiros N, Ribeiro L, Lima N, Oliveira P. Predictive factors of revision endoscopic sinus surgery. Eur Arch Otorhinolaryngol. 2023; 280: 3265-3269.
- Scangas GA, Wu AW, Ting JY, Metson R, Walgama E, Shrime MG, Cost Utility Analysis of Dupilumab Versus Endoscopic Sinus Surgery for Chronic Rhinosinusitis With Nasal Polyps. Laryngoscope. 2021; 131: E26-E33.
- Patel GB, Kudlaty EA, Guo A, Yeh C, Kim MS, Price CPE, et al. Impact of type 2 targeting biologics on acute exacerbations of chronic rhinosinusitis. Allergy Asthma Proc. 2021; 42: 417-424.
- Naclerio R, Mullol J, Stevens WW. A Decade of Clinical Advances in Chronic Rhinosinusitis: 2012-2022. J Allergy Clin Immunol Pract. 2023; 11: 43-50.
- Lipworth BJ, Chan R. The Choice of Biologics in Patients with Severe Chronic Rhinosinusitis with Nasal Polyps. J Allergy Clin Immunol Pract. 2021; 9: 4235-4238.
- Shaw CK, Cowin A, Wormald PJ. A study of the normal temporal healing pattern and the mucociliary transport after endoscopic partial and full-thickness removal of nasal mucosa in sheep. Immunol Cell Biol. 2001; 79: 145-148.
- Blauwblomme M, Georgalas C, Ahmed S, Alobid I, Battaglia P, Castelnuovo P, et al. Expert Consensus on Surgical Management of Primary Diffuse Type 2-Dominant Chronic Rhinosinusitis. Int Forum Allergy Rhinol. 2025; 15: 303-316.
- Canonica GW, Passalacqua G. Disease-modifying effect and economic implications of sublingual immunotherapy. J Allergy Clin Immunol. 2011; 127: 44-45.
- Rudman Spergel AK, Minnicozzi M, Wheatley LM, Togias A. Is Allergen Immunotherapy in Children Disease Modifying? A Review of the Evidence. Curr Allergy Asthma Rep. 2018; 18: 47.
- onekura S, Gotoh M, Kaneko S, Maekawa Y, Okubo K, OkamotoY. Disease-Modifying Effect of Japanese Cedar Pollen Sublingual Immunotherapy Tablets. J Allergy Clin Immunol Pract. 2021; 9: 4103- 4116.e14.
- Korn S, Cook B, Simpson LJ, Llanos JP, Ambrose CS. Efficacy of Biologics in Severe, Uncontrolled Asthma Stratified by Blood Eosinophil Count: A Systematic Review. Adv Ther. 2023; 40: 2944-2964.
- Varricchi G, Ferri S, Pepys J, Poto R, Spadaro G, Nappi E, et al. Biologics and airway remodeling in severe asthma. Allergy. 2022; 77: 3538- 3552.
- Shah PA, Brightling C. Biologics for severe asthma-Which, when and why? Respirology. 2023; 28: 709-721.
- Wolfe SG, Schlosser RJ, Bolger WE, Lanza DC, Kennedy DW. Endoscopic and endoscope-assisted resections of inverted sinonasal papillomas. Otolaryngol Head Neck Surg. 2004; 131: 174-179.
- Miglani A, Patel SH, Kosiorek HE, Hinni ML, Hayden RE, Lal D. Endoscopic resection of sinonasal mucosal melanoma has comparable outcomes to open approaches. Am J Rhinol Allergy. 2017; 31: 200- 204.
- Molteni G, Sacchetto A, Saccardo T, Gulino A, Marchioni D. Quality of Life Evaluation After Trans-Nasal Endoscopic Surgery for Skull Base Tumors. Am J Rhinol Allergy. 2021; 35: 507-515.
- Hopkins C, Slack R, Lund V, Brown P, Copley L, Browne J. Long-term outcomes from the English national comparative audit of surgery for nasal polyposis and chronic rhinosinusitis. Laryngoscope. 2009; 119: 2459-2465.