Satisfactory Clinical Complete Response of Tonsil Squamous Cell Carcinoma with Combined Pembrolizumab and Docetaxel Chemotherapy: A Case Report
- 1. Oncological Department of Oral and Maxillofacial Surgery, The First Affiliated Hospital of Xinjiang Medical University, School/Hospital of Stomatology Xinjiang Medical University, Urumqi 830054, Xinjiang Uygur Autonomous Region, China
- 2. Department of Oncology, Daping Hospital, Army Medical University, 10 Changjiang Zhilu, Daping Yuzhong, Chongqing 400042, China
Keywords
• Clinical complete response
• Multidisciplinary approach
• Neoadjuvant immunotherapy
• Proton heavy ion therapy
• HPV type 16
Abstract
Introduction: Tonsillar Squamous Cell Carcinoma (TSCC) is a common, difficult-to-treat oropharyngeal cancer.
Case summary: A 50-year-old man was diagnosed with left TSCC with multiple lymph node metastases on the left side of the neck (stage cT3N3b). The patient tested positive for human papillomavirus/p16.
Discussion: The patient had a high tumor mutational burden of 14.08 Muts/Mb and positive programmed cell death-ligand 1 expression (15%). A combination therapy of pembrolizumab, docetaxel, cisplatin, and proton and heavy ion therapy was administered. Magnetic resonance imaging after four cycles showed that the left tonsil carcinoma disappeared, and the left cervical lymph node significantly shrank to 1.1 cm, suggesting that the patient achieved a clinical complete response with no signs of disease progression. The patient was administered pembrolizumab every month to maintain for 2 years and followups at the interval of 3 months. The long-term complete clinical response in our case may represent a new combined therapeutic approach for locally advanced unresectable head and neck squamous cell carcinoma.
Citation
Tan X, Li J, Wei W, Chen H, Wang S, et al. (2024) Satisfactory Clinical Complete Response of Tonsil Squamous Cell Carcinoma with Combined Pembrolizumab and Docetaxel Chemotherapy: A Case Report. Ann Otolaryngol Rhinol 11(5): 1348.
INTRODUCTION
Tonsillar Squamous Cell Carcinoma (TSCC) is one of the most common oropharyngeal cancers. The clinical treatment of patients with cervical lymph node metastasis is challenging, especially when the metastatic cervical lymph nodes diameter exceeds 6 cm, indicating poor prognosis. Over 50% of patients with locally advanced Head and Neck Squamous Cell Carcinoma (HNSCC) have recurrence or develop metastases within 3 years of traditional treatments [1]. Therefore, it is clinically significant to explore more effective strategies to improve patient prognosis.
Herein, we report a case of initially unresectable TSCC that was treated with a combination of pembrolizumab, proton heavy ion therapy, and chemotherapy and achieved a long-term clinical Complete Response (CR) with no sign of disease progression. Furthermore, the function of the target organ was maintained, which greatly improves the patient’s quality of life.
CASE PRESENTATION
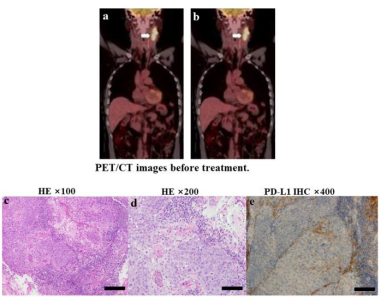
A 50-year-old man with no history of smoking or alcohol consumption was admitted to a local hospital with a mass on the left side of the neck on May 14, 2020. B-type ultrasonography revealed an enlarged lymph node on the left side of the neck. Subsequently, a biopsy of the left cervical lymph node was performed. The patient was transferred to our hospital on May 31, 2020. Positron emission computed tomography revealed a tumor (diameter, 49 mm) on the left tonsil [Figure 1a] and a metastatic lymph node (diameter, 70 mm) on the left side of the neck [Figure 1b] with no distant metastatic lesions. A biopsy of the tumor on the left tonsil confirmed the primary diagnosis of squamous cell carcinoma with human papillomavirus and p16 positivity [Figure 1c,1d]. Molecular tests of the peripheral blood and lesion tissue showed a high Tumor Mutational Burden (TMB) of 14.08 muts/ Mb [Table 1] and a positive PD-L1 expression of 15% [Figure 1e]. Based on the results, the tumor stage was cT3N3bM0 IVB (American Joint Committee on Cancer 8th Cancer Staging Manual 2017), which was unsuitable for surgical resection.
Table 1: Genetic analysis of the left tonsil tumor and blood.
|
Gene |
Nucleotide change |
Amino acid change |
Mutation effect |
Left tonsil tumor VAF (%) |
|
ARID1B |
c.4010G>A |
p.R1337Q |
Nonsynonymous |
8.1 |
|
BAP1 |
c.163G>A |
p.E55K |
Nonsynonymous |
9.8 |
|
BCORL1 |
c.2218C>T |
p.R740C |
Nonsynonymous |
6.6 |
|
CD70 |
c.488G>A |
p.R163Q |
Nonsynonymous |
8.9 |
|
CHD2 |
c.218C>A |
p.S73Y |
Nonsynonymous |
17.1 |
|
CREBBP |
c.2402C>A |
p.S801* |
Nonsynonymous |
1.2 |
|
DDX3X |
c.137G>A |
p.R46Q |
Nonsynonymous |
6.4 |
|
ERBB3 |
c.3886C>T |
p.Q1296* |
Nonsynonymous |
16.7 |
|
F8 |
c.6683G>C |
p.R2228P |
Nonsynonymous |
6.3 |
|
FGF19 |
c.240G>A |
p.E81K |
Nonsynonymous |
5.1 |
|
FGFR4 |
c.826G>A |
p.D276N |
Nonsynonymous |
15.9 |
|
FLT1 |
c.3237G>C |
p.K1079N |
Nonsynonymous |
8.2 |
|
HISTIH3I |
c.395G>C |
p.R132P |
Nonsynonymous |
6.1 |
|
KMT2E |
c.3338G>C |
p.R1113T |
Nonsynonymous |
8.3 |
|
LATS1 |
c.281C>A |
p.S94Y |
Nonsynonymous |
17.3 |
|
MACF1 |
c.9013G>C |
p.D3005H |
Nonsynonymous |
10.3 |
|
NFATC2 |
c.431C>T |
p.S144L |
Nonsynonymous |
12.2 |
|
PDGFRB |
c.2923G>A |
p.E975K |
Nonsynonymous |
8.3 |
|
PIK3CA |
c.1633G>A |
p.E545K |
Nonsynonymous |
13.5 |
|
PTPRD |
c.997G>A |
p.E333K |
Nonsynonymous |
7.2 |
|
SESN2 |
c.655G>C |
p.E219Q |
Nonsynonymous |
9.8 |
|
SMC1A |
c.1154G>C |
p.R385T |
Nonsynonymous |
7.0 |
|
STAT1 |
c.574C>T |
p.Q192* |
Nonsynonymous |
8.5 |
|
TAF1 |
c.2986C>T |
p.R996C |
Nonsynonymous |
30.7 |
|
TAP1 |
c.1433C>A |
p.S478* |
Nonsynonymous |
10.5 |
|
MSI |
|
|
|
MSS |
|
TMB |
|
|
|
14.08 mutations/MB |
Figure 1: PET/CT images before treatment. a: A 49 mm-diameter tumor in the left tonsil b: An enlarged metastatic lymph node with a diameter of approximately 70 mm on the left side of the neck. PET/CT, positron emission tomography/computed tomography; Pathologic findings in a biopsy of the neoplasm in the left tonsil indicated HPV-related squamous cell carcinoma and PD-L1 expression; c: HE staining × 100; d: HE staining × 200; e. PD-L1 expression of 15%. Abbreviations: HPV: Human Papillomavirus; PF-L1: programmed cell death-ligand 1; HE: Hematoxylin and Eosin.
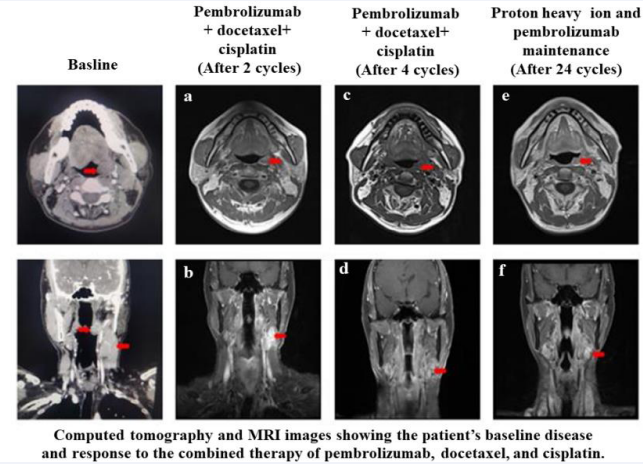
To downstage the tumor and render it resectable, the patient received four cycles of neoadjuvant therapy (pembrolizumab 200 mg, docetaxel 75 mg/m2 , and cisplatin 75 mg/m2 every 3 weeks). After two cycles of treatment, the contrast-enhanced Magnetic Resonance Imaging (MRI) of the maxillofacial region showed that the left tonsil had significantly shrunk, with no obvious space-occupying or enhancing lesions [Figure 2a], and a 2.3 × 1.1 × 3.4 cm irregular mass of soft tissue was observed in the deep space of the left neck [Figure 2b]. After four cycles, the MRI scan demonstrated that the lesions on the left tonsil disappeared [Figure 2c], and the left cervical lymph node reduced to 11 mm in diameter [Figure 2d].
Figure 2 Computed tomography and MRI images showing the patient’s baseline disease and response to the combined therapy of pembrolizumab, docetaxel, and cisplatin. After two cycles of treatment: a: the left tonsil was slightly enlarged but with no obvious space-occupying or enhancing lesions; b: 2.3 × 1.1 × 3.4 cm irregular mass of soft tissue was observed in the deep space of the left neck. After four cycles of treatment: c: the tumor in the left tonsil disappeared, with no obvious abnormal enhancement; d: the left cervical lymph node was reduced to 11 mm in diameter. The patient achieved a complete clinical response. After proton heavy ion and pembrolizumab maintenance: e: the tumor in the left tonsil disappeared; f: the left cervical lymph node was 11 mm in diameter. The patient achieved long-term benefits after maintenance treatment with pembrolizumab 200 mg per month.
Abbreviations: MRI: Magnetic Resonance Imaging.
DISCUSSION
A case management meeting was conducted by a Multidisciplinary Team (MDT) to discuss whether surgical resection should be performed. The patient was informed of all the necessary information in the MDT meeting, and he discontinued the plan for surgery. Thereafter, the patient underwent proton heavy ion therapy at the Shanghai Proton Heavy Ion Hospital on September 8, 2020. The left tonsil and metastatic lymph node area of the neck were radiated. Subsequently, the patient underwent two cycles of combined therapy (pembrolizumab 200 mg, docetaxel 75 mg/m2 , and cisplatin 75 mg/m2 every 3 weeks). To date, the patient has received an intravenous injection of pembrolizumab 200 mg every month as maintenance immunotherapy. The patient was followed-up every 3 months with no signs of disease progression [Figure 2e, 2f].
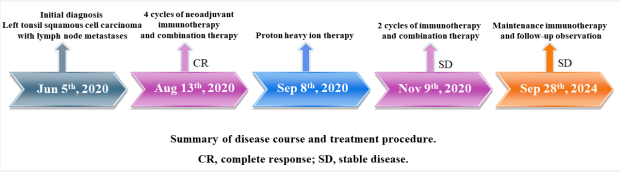
The timeline of the patient’s treatment is shown in Figure 3. No adverse events were observed during the treatment (according to the National Cancer Institute’s Common Terminology Criteria for Adverse Events, version 4.0) [2]. The traditional treatment options for TSCC include surgery, chemotherapy, radiation therapy, and targeted therapy [3]. However, the efficacy of these treatments remains limited, necessitating the development of novel therapies. Accumulating studies have shown that immune checkpoint inhibitors can be used as second-line, first-line, and neoadjuvant therapies and have proven effective against solid tumors.
However, many patients are unable to benefit from these drugs, resulting in a low overall response rate. To date, there are no validated predictive immune biomarkers with unified standards for all patients with HNSCC, although many promising candidate biomarkers are being investigated. In our case, molecular tests of the peripheral blood and lesion tissue showed a high TMB of 14.08 muts/Mb [Table 1] and a positive PD-L1 expression of 15%. Based on these results, the patient was treated with combined immunotherapy and achieved complete long-term clinical remission with no signs of disease progression. Intensity-modulated conformal radiotherapy (SBRT) is indispensable for locoregionally advanced unresectable HNSCC.
Proton heavy ion therapy is an advanced method of radiotherapy used as a radical treatment for various malignant tumors. Clinical trials of proton heavy ion therapy are underway, suggesting its potential to replace SBRT [4]. Based on the advantages of protons and heavy ions, our patient discontinued SBRT and underwent proton heavy ion therapy at the Shanghai Proton Heavy Ion Hospital. Some studies have shown that proton heavy ion therapy increases immune-recognized surface molecules expression and the sensitivity of tumor cells to cytotoxic T-lymphocyte killing [5]. In our case, the initially unresectable TSCC treated with the combined use of pembrolizumab, proton heavy ion therapy, and chemotherapy achieved a long-term clinical CR with no signs of disease progression. Moreover, no adverse events were observed during treatment.
CONCLUSION
To the best of our knowledge, this is the first case of unresectable TSCC after immunotherapy combined with proton heavy ion therapy and chemotherapy that achieved long-term clinical complete remission with no sign of disease progression, which implies the potential value of combined immunotherapy for locally advanced unresectable HNSCC. However, the treatment of this patient had one limitation. Since the patient achieved complete clinical remission, we repeatedly recommended a pathological biopsy of the left tonsil to observe whether complete pathological remission was achieved. However, the patient refused to undergo a case biopsy again; therefore, we could not obtain pathological pictures to evaluate whether the patient had achieved pathological complete remission.
ACKNOWLEDGMENTS
We express our deepest appreciation for the Tianshan Innovation Team of Xinjiang Uygur Autonomous Region for approving and supporting the study (grant number: 2021D14001).
We also thank Editage (app.editage.cn) for providing linguistic assistance during the preparation of this manuscript.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors upon reasonable request.
REFERENCES
- Cohen EEW, Soulières D, Le Tourneau C, Dinis J, Licitra L, Ahn MJ, et al. Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head-and-neck squamous cell carcinoma (KEYNOTE-040): A randomised, open-label, phase 3 study. Lancet. 2019; 393: 156–67.
- Bennett AV, Dueck AC, Mitchell SA, Mendoza TR, Reeve BB, Atkinson TM, et al. Mode equivalence and acceptability of tablet computer-, interactive voice response system-, and paper-based administration of the U.S. National Cancer Institute’s Patient-Reported outcomes version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE). Health Qual Life Outcomes. 2016; 14: 24.
- Nutting CM, Morden JP, Harrington KJ, Urbano TG, Bhide SA, Clark C, et al. Parotid-sparing intensity modulated versus conventional radiotherapy in head and neck cancer (PARSPORT): a phase 3 multicentre randomised controlled trial. Lancet Oncol. 2011; 12: 127–36.
- Durante M, Loeffler JS. Charged particles in radiation oncology. Nat
-
Rev Clin Oncol. 2010; 7: 37–43.Gameiro SR, Malamas AS, Bernstein MB, Tsang KY, Vassantachart A, Sahoo N, et al. Tumor cells surviving exposure to proton or photon radiation share a common immunogenic modulation signature, rendering them more sensitive to T cell-mediated killing. Int J Radiat Oncol Biol Phys. 2016; 95: 120–30.