The Human Mandible - Embryological, Anatomical, and Pathological Aspects
- 1. Department of Odontology, University of Copenhagen, Denmark
Abstract
Human developmental disorders can be inborn or disrupted disorders. In both conditions insights in the steps in normal embryological development is necessary for postnatal based diagnostics.
Keywords
• Embryology; Mandibular development; Radiological methods
CITATION
Kjær I (2024) The Human Mandible – Embryological, Anatomical, and Pathological Aspects. Ann Otolaryngol Rhinol 11(3): 1335.
INTRODUCTION
This present overview of the human mandible gives information on the steps in normal human mandibular development prenatally, in the postnatal anatomical development and exemplify how postnatal pathological cases can be understood from normal pattern in development The results are based on histological, anthropological and radiological methods performed on human material.
The studies were inspired by professor odont.dr. Arne Bjørk, who in 1970 wanted histological research on human material to be included in the research profile at the institute of orthodontics in Copenhagen. With that purpose he arranged a 5-year research grant for relevant human studies. The only systematic human tissue assessable in 1970 was the human prenatal mandible at the anatomy department at the University of Copenhagen. How this tissue could be relevant for clinical orthodontics remained a question at that time. But remembering the sentence from Winston Churchill:” The further you can look back, the further you can look ahead” encouraged the project which resulted in an odontological doctoral thesis on the human mandibular embryology in 1982 [1].
Later admission was given to other craniofacial regions and to study fetal pathology, which resulted in a medical doctoral thesis in 1999 [2]. At that time the relevance for insight in normal embryology was obvious not only for fetal pathology but also for postnatal pathology.
EMBRYOLOGICAL DEVELOPMENT OF THE HUMAN MANDIBLE
Steps in normal embryological development of the human mandible was studied radiographically, histologically, and anthropologically until about age 20 weeks of Gestation. Mandibles from human fetuses older than 20 weeks of Gestation underwent anthropological examination.
These steps include the following: Cartilage/ bone development from neural crest cells, tooth formation and the alveolar process, the mental foramen, symphysis menti, physiological movements of the bony mandibular corpus, development of the mandibular condyle, innervation and formation of the mandibular canal and mandibular growth prenatally.
Cartilage/ bone development from neural crest cells
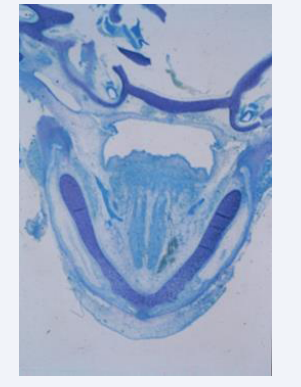
The bilateral Meckels cartilages are the first histological signs of the developing mandible [Figure 1] [3].
Figure 1: Horizontal histological section of a human mandible Gestational age 7 weeks. The bilateral Meckel’s cartilages are marked M. Bone formation has seemingly not started.
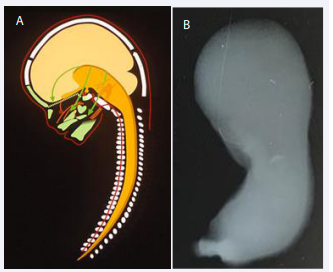
The cells forming the mandible have migrated from the neural crest as demonstrated schematically in Figure 2A. The Bony development of the claviculae are the first bones to ossify in the human body, shortly followed by ossification of the mandible [Figure 2B]. The bony formation starts in the canine region. Anterior to this region the mandible developed cartilaginously and posterior to that region the mandible developed membranously [Figure 3] [3].
Figure 2 A: Schematic drawing of the human body axis demonstrating the origin of the vertebral column (illustrated by red marking of the notochord in the corpora of the vertebrae) and the green craniofacial bones, originating from the dark yellow neural tube. From the neural crest of the neural tube the multipotent cells migrate anteriorly to form the craniofacial skeleton as demonstrated by green arrows. Note that cells from different sites on the neural tube migrates to different bony regions. B: Illustration of a whole-body radiograph from a human fetus,7 weeks of Gestation. The radiograph has been taken in a specific bone- sensitive X-ray machine The right arrow indicates ossification of the clavicular bone, which is the first bone in the human body to ossify. The left arrow marks the region in which the mandible starts ossification, shortly after start of ossification in the clavicula.
Figure 3: Horizontal histological section of a human mandible, Gestational age 9 weeks. The bony components are black due to silver impregnation. The arrow marks the symphysis menti region in the mid-axis of the body. The rostral parts of the purple Meckel’s cartilage are undergoing ossification. Immature tooth buds are observed in the upper part of the figure.
Tooth formation and development of the alveolar process
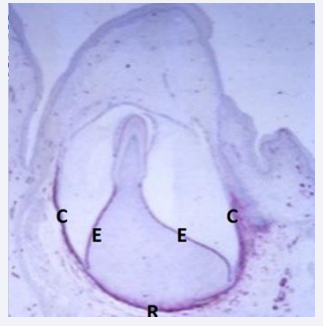
The primary dentition develops early from the dental lamina. The tooth buds consist before Gestional age 20 of early dentin and enamel formation in the coronal part of the surrounding dental follicle which is highly innervated apically [Figure 4] [4].
Figure 4: Illustration of an early stage in development of a primary tooth. C marks the crown follicle and R the root membrane. E marks the inner enamel epithelium and the red color indicates reactions for peripheral nerves ( NGFR, Nerne Growth Factor Receptor reaction) Dentine and enamel are not clearly registered at this stage.
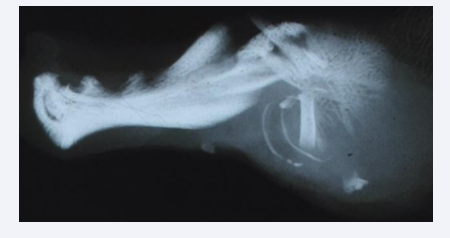
Alveolar processes develop in accordance with the tooth buds. In the region of the primary canine and in the first primary molar region the facial alveolar process is late in development [Figure 5] [5,6].
Figure 5: Radiograph taken by a bone sensitive X-ray Machine ( Faxitron Machine) demonstrating a lateral view of a human fetal mandible about 15 weeks of Gestation. The arrows mark the cavities where the primary canine and first molar were located . Between these cavities interdental alveolar bone has developed.
The mental foramen
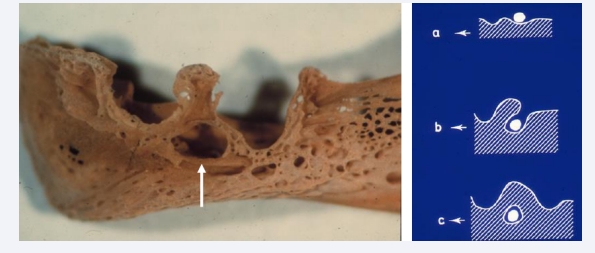
The Mental foramen develop between the primary canine and first molar tooth buds by bony tissue gradually encircling the mental nerve [Figure 6] [7,8].
Figure 6: Left: Eviscerated human fetal mandible, age 15 weeks of Gestation. The arrow marks the mental foramen between the open alveolar structures where the primary canine and first primary molar were formed. Right: Three drawings demonstrating how the mental foramen was formed by bone encircling the peripheral nerve (round white structure) from stage a to c.
Symphysis menti
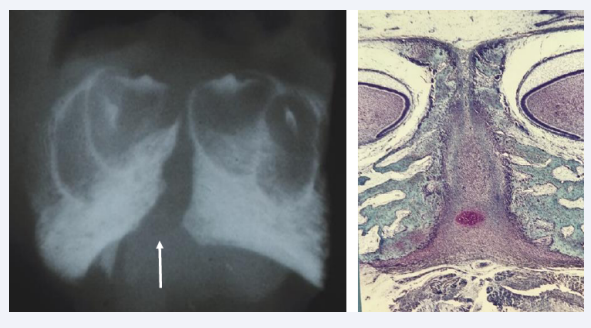
The Symphisis menti located midaxially in the body constitutes of a central core of mesenchymal tissue, first bordered by bilateral Meckels cartilage and gradually by bony tissue anteriorly and later also by bony tissue posteriorly. By immunohistochemical reactions heavy growth activity was registered in the symphysis menti [Figure 7] [3].
Figure 7: Left: Frontal radiograph of the mental region in a human fetus19 weeks of Gestation. The radiograph taken with a bone sensitive x-ray machine demonstrates the Symphysis Menti (arrow) and bony alveolae for the primary incisors, where dentine and enamel formation have started. Right: Frontal histological section of the specimen demonstrated in the left figure. The broad Symphysis menti is registered, richly vascularized, and bordered by bilateral bone tissue with tooth buds located in alveoli.
Physiological movements of the early mandibular bone
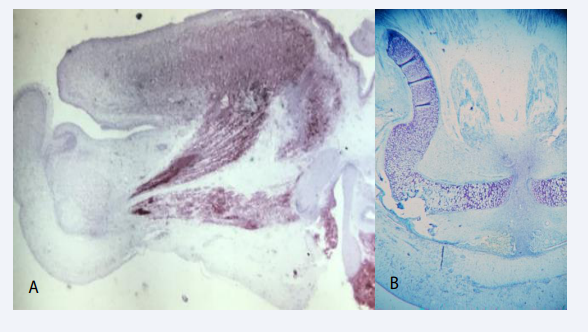
Before development of the mandibular condyle the bilateral bony mandibular components suddenly changed position caused by a muscular contraction by the musculus genio-glossis and musculus genio-hyoideus [Figure 8A].
Figure 8 A: Mid-sagittal section through the tongue in a human fetus 12 weeks of Gestation. The red color express NGFR ( Nerve growth Factor Receptor) indicating muscle activity. Two muscle bundles appear active. The upper is the genioglossus muscle and the lower the geniohyoid muscle. Contraction of these two muscles results in a backward and downward move of the tongue and mandible. These physiological processes are responsible for creating space for the maxillary shelves to elevate and fuse during palate formation. B: Histological horizontal section of the mandible, Gestational age 13 weeks, demonstrating the S-shape of the Mekel’s cartilage, which is a result of the backward drawing of the mandible by muscular traction (see figure A).
This contraction moves the jaw components backward resulting in an enlarged distance between the distal parts of the mandibular components [9-11]. This movement occur exactly when the maxillary palatal shelves change from a vertical to a horizontal position, forming the palate. This backward movement of the tongue and mandible necessary for forming the palate can be observed in the S-shape of the Meckels cartilage [Figure 8B].
Development of the mandibular condyle
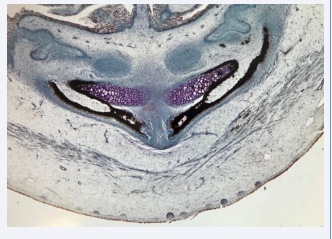
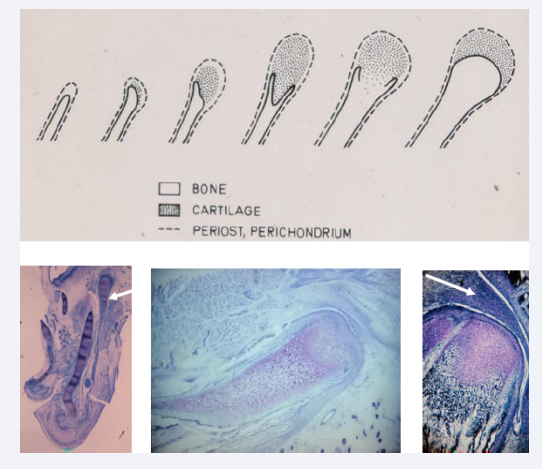
The mandibular condyle is formed after the backward movement of the mandibular components. Cartilaginous cells appear disto-laterally on the mandibular bone [Figure 9A] and gradually the cartilage grows and changes from carrot shape to bean shape, covering a bony surface [Figure 9B, Figure 9C]. In the cartilaginous cell layer canals can be observed [Figure 9D]. Also, the germinative cell layer important for condylar growth appear when joint cavity and discus have developed [9,12-14].
Figure 9: In the upper part of this figure drawings indicate steps in development of the mandibular condyle. In the lower part three histological sections in different slanting planes illustrate the location and morphology of the mandibular condyle from gestational age 15-20 weeks. Lower left figure demonstrates that the condyle (white arrow) develops laterally compared to the Mekel’s cartilage. In the center a vertical section demonstrates the length of the condylar cartilage before resorption starts. This figure also demonstrates development of the lower cavity in the jaw joint and the early formation of the discus. The right section in the lower part of the figure demonstrates a canal in the condylar cartilage and a fully developed joint cavity with a discus marked with a white arrow
Innervation and formation of the mandibular canal
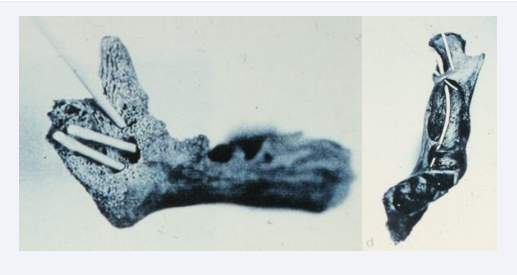
The mandibular canal was first observed on anthropological material after 20 weeks of age. The nerve fibers in the mandibular inferior nerve were gradually encircled by bone tissue, resulting in formation of the mandibular canal [15-18]. The first peripheral nerves which was encircled was the nerves to the incisors located in the lower aspect of the canal and these were followed by the nerves to the canines and molars located in the upper aspects of the canal [Figure 10].
Figure 10: Two human anthropological mandibles from about 30 weeks of Gestation illustrate the initial formation of the alveolar inferior nerve and the mandibular canal. Left mandible is observed from the lingual side with gutta-percha points ( root filling sticks) resembling peripheral nerves inserted in different holes. Right mandible observed from above. In this figure the gutta-percha points indicate that the peripheral nerves have different directions and are located in different levels.
Mandibular growth Prenatally
As a conclusion the growth of the prenatal mandible was registered in the sagittal, vertical, and transversal planes [19,20]. The sagittal growth occurred in the condyle and the vertical growth as well in the condyle as in the alveolar processes. The transversal growth occur in the symphysis menti and also in the mandibular condyle .
ANATOMICAL DEVELOPMENT OF THE HUMAN MANDIBLE
During childhood the mandibular size, shape, and specific bony structures changes in individual patterns. Also, the dentition with primary and permanent teeth develops during childhood, early puberty and adolescence. The postnatal mandible is mostly studied on radiographs and in anthropological cases. Histological investigations on human postnatal mandibles are very seldom. In the following specific bony structures are highlighted.
Symphysis Menti
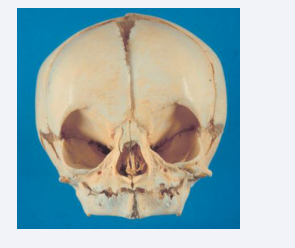
The symphysis menti is still an open symphysis at birth [Figure 11]. The exact time for closure is not known, but probably about 1year of age [17].
Figure 11: Frontal view of a human cranium from a child aged 0-6 month. In the mandible the symphysis menti is open and the alveolar process not developed.
Mandibular condyle and the coronoid process
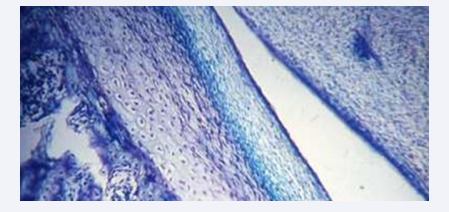
The mandibular condyle appears histologically with different cell layers at birth. These layers are inner layers of cartilage which is immature towards the joint cavity. The cartilage layers are covered by a germinative vascularized cell layer covered by a fiber layer towards the joint cavity [Figure 12]. The joint cavity appears with a discus separating the joint cavity in a lower and upper joint cavity [Figure 12] [17]. The mandibular condyle is an important center for mandibular growth in the sagittal, vertical, and transversal planes [19,20].
Figure 12: Histological section from a child illustrating the tissue layers in the mandibular condyle. The arrow marks the germinative cell layer, where cell renewal and condylar growth occur, This layer is located between the cartilage and a fiber layer towards the joint cavity.
Lingulae mandibulae
The lingula mandibulae has developed at birth. This bony structure covers the mandibular foramen which is the entrance to the mandibular canal [17].
Mandibular canal, normal growth pattern
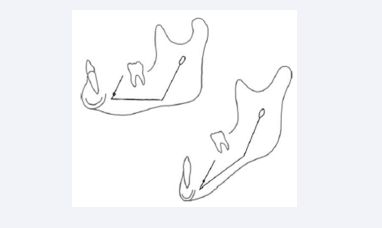
The mandibular canal has a straight morphology at birth. Later in childhood the canal appears curved. This curvature seems comparable to the outer jaw morphology, expressed as the angle formed by the posterior contour of the mandibular ramus and the line marking the lower contour of the corpus mandibulae [Figure 13] [21].
Figure 13: Drawings of two different mandibles with different morphologies observed from the lateral aspect. In both mandibles contours of the mandibular canal are inserted. The curvature of the canal resembles the outer mandibular contour expressed as the ramus/ corpus angle.
Mental canal normal growth
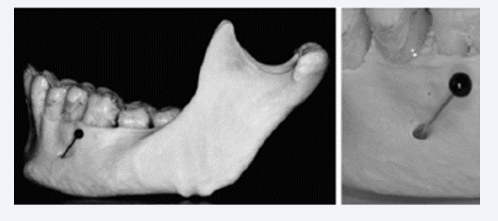
The mental foramen is postnatally located between the first and second primary molars. The postnatal mental foramen forms the entrance to a mental canal which has developed in the late prenatal period. This canal has different orientations in different jaw morphologies [Figure 14] [21].
Figure 14: canal demonstrated in an adult anthropological mandible. This canal has developed during mandibular growth by bone apposition. The canal has different directions depending on the growth pattern.
Cephalometry: purpose, methods, and results.
Postnatal mandibular morphologies are investigated by the cephalometric method. By using radiological landmarks, the mandibular morphology is defined in the sagittal, vertical, and transversal planes. The purpose of performing the cephalometric method is to predict the growth pattern of the mandible and occlusion, for optical orthodontic treatment. Cephalometry has during decades been an important tool in orthodontic diagnostics. Cephalometry methods have documented by Björk and Skieller [22] that the mandible during growth not only move in the anterior direction but also rotate upward, resulting in a deep bite or downward resulting in an open bite.
Tooth development, and eruption pattern.
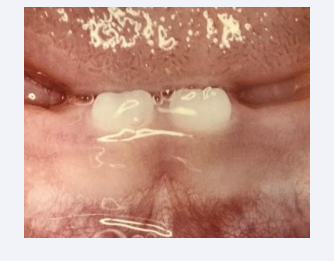
The primary dentition continues development postnatally. The first teeth to erupt in the mouth are the mandibular primary central incisors [Figure 15]. These teeth erupt about the age of 9 months and shedded when the first permanent central incisors erupt at the age of 7 years. There are specific times for eruption and shedding of the primary teeth. The primary molars are replaced by permanent premolars. The permanent molars develop independent of the primary dentition.
Figure 15: The first teeth to erupt are the primary mandibular central incisors erupting at about 9 months of age.
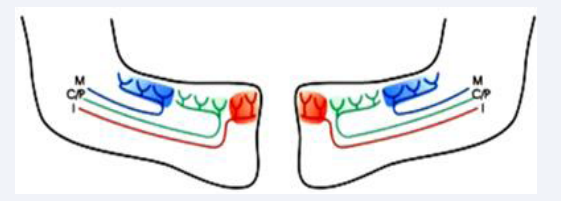
The innervation has been demonstrated to be the driving force behind the eruptive process [23]. This driving force depends on the peripheral nerve branches in the mandibular canal [24,25]. The different nerve branches innervate different tooth groups [Figure 16]. The innervation pattern explains the sequence in eruption. The innervation might also indicate that the mandibular growth pattern is controlled by peripheral nerves [26].
Figure 16: Schematic drawings of the left and right half part of the mandibular bone. Inserted in colors are the innervation to the incisors, I (red) to the canine, premolars C/P (green) and molars M (blue)
EXAMPLES OF PATHOLOGICALHUMAN MANDIBLES EVALUATED FROM PRENATAL INSIGHT
The following structures and morphologies in the pathological developed mandible will be described:
Symphysis menti not closed
It appears very seldom that a seemingly pathological process prohibit closure of the symphysis menti [17].
Abnormal mental foramen
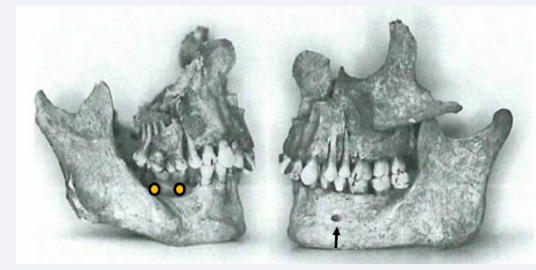
Abnormal morphologies of the mental foramen with closed appearance have been studied on anthropological mandibles. This is a sign indicating agenesis of the premolars innervated from the mental nerve. This condition is demonstrated in Figure 17 [17,27].
Figure 17: Right and left sides of the maxilla and mandible from the same anthropological case. The arrow marks presence of the mental foramen in the left side, while the mental foramen is absent in the right side where also agenesis of the premolars occurs (yellow dots).
Mandibular condylar joint anchylosed
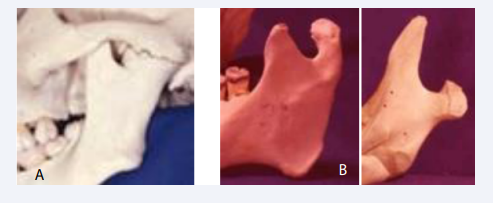
Mandibular joins anchylosed to the cranial base prohibit mandibular jaw movements. This condition might be a sequala after an infection, is shown in Figure 18A.
Figure 18: A: Illustration of a mandibular condyle anchylosed in the joint cavity to the cranial base. B: Two examples of malformed mandibular condyles. In the right case severe elongation of the coronoid process is observed.
Mandibular condyle abnormalities
Inborn morphological abnormalities of the condylar processes are demonstrated in Figure 18B.
Absence of the mandibular canal
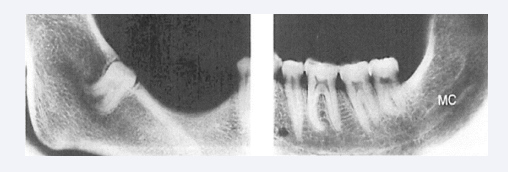
Absence of the entire mandibular canal or parts of the mandibular canal are morphologies seldom observed in mandibles with unilateral complete or partial agenesis of teeth [Figure 19] [27].
Figure 19: Radiographs of right and left sides in an anthropological mandible lacking mandibular canal in the right side. In the left side is the canal present and indicated MC.
Mandibular tooth agenesis
Mandibular tooth agenesis, (not formed) occur nearly always in mandibles with normal mandibular canals. The third molar (wisdom tooth) and the second premolars are the teeth most frequently missing. Agenesis of the second mandibular premolar can occur in combination with agenesis of the second maxillary premolars [28]. The teeth which very seldom are missed are the incisors, the canines and the first permanent molars . Agenesis occurs rarely in the primary dentition [29-32].
Abnormal mandibular tooth eruption
Two types of abnormal eruption are frequent in the mandible. These are arrested eruption and ectopic eruption.
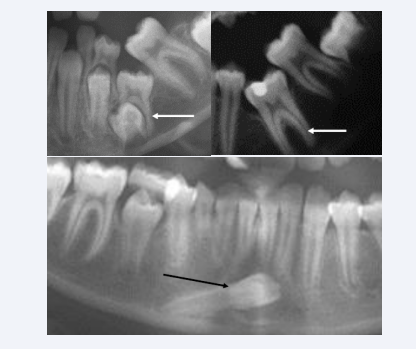
Arrested eruption occur in the primary and permanent dentitions. The etiology behind is often anchylosis-but why anchyloses has developed is not known [33-38]. Ankylotic conditions of a primary molar and of a permanent molar are demonstrated in Figure 20A.
Figure 20: Sections from three orthopantomograms illustrating abnormal development of mandibular teeth. Upper radiographs demonstrate anchylosed primary molar to the left and anchylosed permanent molar to the right. The anchylosed teeth are marked by white arrows. Lower radiograph illustrates ectopically permanent mandibular canine located nearly horizontally in the mandibular bone.
Ectopia can be registered in all mandibular permanent teeth. The most seldom type of ectopia seems to be mandibular canine ectopy shown in Figure 20B [39,40]. Several studies on arrested eruption and ectopic eruption have been published with discussion of etiology.
Space problems for dental eruption
Space problems for the third molars can be registered and possibly predicted in orthopantomograms and profile radiographs [41].
Syndromes affecting the mandibular bone.
In cases with ectodermal dysplasia and agenesis of more than 13 teeth the mandible appears retrognathic [42]. Several other syndromes appear with bony defects or abnormal growth pattern [43,44].
Craniofacial surgery in the mandible
Craniofacial surgery is a specialty in odontology involving surgery in three planes of the mandibular bone including the alveolar process and condyle. This reconstruction occurs in trauma cases and in cases where surgery is needed for corrections of severe malocclusions.
CONCLUSION
This presentation summarizes several out of many investigations performed in a 50-year period. During these years the prenatal human mandible was the first material which was offered to study at the medical institute at the University of Copenhagen. Later material from different hospitals gave assess to fetal pathological material. During the entire research period several other aspects in prenatal craniofacial development were studied and new fetal pathological insights were added to fetal pathological diagnostics. Studies during this 50-year period gave inspiration to understand the interrelationship between bone, peripheral nerves, and the central nervous system [45- 50]. As physiological processes in the craniofacial area are also dependent on the nervous system, this overview might inspire new research collaborations between otolaryngology, Rhinology and Odontology.
REFERENCES
- Kjær I. Prenatal development of the mandible in humans. Doctoral Thesis, The Royal Dental School, Copenhagen DK, 1982.
- Kjær I. Neuro-osteology. Crit Rev Oral Biol Med. 1998; 9: 224-44.
- Kjær I. Histochemical investigations on the symphysis menti in the human fetus related to fetal skeletal maturation in the hand and foot. Acta Anat (Basel). 1975; 93: 606-33.
- Kjær I. Development of deciduous mandibular incisors related to developmental stages in the mandible. Acta Odontol Scand. 1980; 38: 257-62.
- Kjær I. Correlated appearance of ossification and nerve tissue in human fetal jaws. J Craniofac Genet Dev Biol. 1990; 10: 329-36.
- Kjær I, Kocsis G, Nodal M, Christensen LR. Aetiological aspects of mandibular tooth agenesis - focusing on the role of nerve, oral mucosa, and supporting tissues. Eur J Orthod. 1994; 16: 371-5.
- Kjær I. Formation and early prenatal location of the human mental foramen. Scand J Dent Res. 1989; 97: 1-7.
- Kjær I, Bagheri A. Prenatal development of the alveolar bone of human deciduous incisors and canines. J Dent Res. 1999; 78: 667-72.
- Kjær I. Human prenatal palatal shelf elevation related to craniofacial skeletal maturation. Eur J Orthod. 1992; 14: 26-30.
- Kjær I. Mandibular movements during elevation and fusion of palatal shelves evaluated from the course of Meckel’s cartilage. J Craniofac Genet Dev Biol. 1997; 17: 80-85.
- Takashi O, Hansen BF, Nolting D, Kjær I. Nerve Growth Factor Receptor Immunolocalization During Human Palate and Tongue Development. Cleft Palate Craniofac J. 2003; 40: 116-25.
- Kjaer I. Histochemical and radiologic studies of the human fetal mandibular condyle. Scand J Dent Res. 1978; 86: 279-299.
- Kjær I. Relation between symphyseal and condylar developmental stages in the human fetus. Scand J Dent Res. 1978; 86: 500-502.
- Bach-Petersen S, Kjær I, Hansen BF. Prenatal development of the human osseous emporomandibular region. J Craniofac Genet Dev Biol. 1994; 14: 135-143.
- Chavéz-Lomelí ME, Mansilla Lory J, Pompa JA, Kjær I. The human mandibular canal arises from three separate canals innervating different tooth groups. J Dent Res. 1996; 75: 1540-1544.
- Kjær I. Prenatal traces of aberrant neurofacial growth. Acta Odontol Scand. 1998; 56: 326-330.
- Kjær I. Etiology-bases dental and craniofacial diagnostics. Wiley Blackwell 2016; 265.
- Kjær I. Human prenatal craniofacial development related to brain development under normal and pathologic conditions. Acta Odontol Scand 1995; 53: 135-143.
- Van den Eynde B, Kjær I, Solow B, Graem N, Kjær T, Mathiesen M. Cranial base angulation and prognathism related to cranial and general skeletal maturation in human fetuses. J Craniofac Genet Dev Biol. 1992; 12: 22-32.
- Eriksen E, Bach-Petersen S, van den Eynde B, Solow B, Kjær I. Midsagittal dimensions of the prenatal human cranium. J Craniofac Genet Dev Biol 1995; 15: 44-50.
- Pálsson SR, Kjær I. Morphology of the mandibular canal and the angulation between the mandibular and mental canals in dry skulls. Eur J Orthod. 2008; 31: 59-63.
- Björk A, Skieller V. Normal and abnormal growth of the mandible. A synthesis of longitudinal cephalometric implant studies over a period of 25 years. Eur J Orthod. 1983; 5: 1-46.
- Kjær I. Mechanism of Human tooth eruption: Review article including a new theory for future studies on the eruption process. Scientifica. 2014; 2014: 341905.
- Parner ET, Heidmann JM, Kjær I, Væth M, Poulsen S. Biological Interpretation of the Correlation of Emergence Times of Permanent Teeth. J Dent Res. 2002; 81: 451-454.
- Kjær I, Nolting D. The human periodontal membrane - focusing on the spatial interrelation between the epithelial layer of Malassez, fibers, and innervation. Acta Odontol Scand. 2009; 67: 134-138.
- Kjær I, Nolting D. Immunohistochemical PGP 9.5 positivity in human osteoblasts may indicate that compensatory and dysplastic craniofacial growth are under control by peripheral nerves. Orthod Craniofac Res. 2008; 11: 196-200.
- Jakobsen J, Jørgensen JB, Kjær I. Tooth and bone development in a Danish medieval mandible with unilateral absence of the mandibular canal. Am J Phys Anthropol. 1991; 85: 15-23.
- Kenrad Breum J, Jarle Christensen i, Kjær I. Gender difference in patterns of second premolar agenesis observed in 4,756 individuals. Eur Archs Paediatr Dent. 2013; 14: 397-403.
- Kjær l. How can cranial bones and teeth in children with craniofacial anomalies indicate disturbances in the brain and cranial nerves. Ann Pediatr Chid Health. 2020; 8: 1188.
- Kjær I, Daugaard-Jensen J. Interrelation between fusions in the primary dentition and agenesis in the succedaneous permanent dentition seen from an embryological point of view. J Craniofac Genet Dev Biol. 2000; 20: 193-197.
- Daugaard S, Christensen IJ, Kjær I. Delayed dental maturity in dentitions with agenesis of mandibular second premolars. Orthod Craniofac Res. 2010; 13: 191-196.
- Kjær I. New diagnostics of the dentition on panoramic radiographs- focusing on the peripheral nervous system as an important aetiological factor behind dental anomalies. Orthodontic Waves. 2012; 71: 1-16.
- Vedtofte H, Andreasen JO, Kjær I. Arrested eruption of the permanent lower second molar. Eur J Orthod. 1999; 21: 31-40.
- Nielsen SH, Becktor KB, Kjær I. Primary retention of first permanent mandibular molars in 29 subjects. Eur J Orthod. 2006; 28: 529-534.
- Kjær I, Fink-Jensen M, Andreasen O. Classification and sequelae of arrested eruption of primary molars. Int J Paed Dent. 2008; 18: 11-17.
- Kjær I, Nielsen MH, Skovgaard LT. Can persistence of primary molars be predicted in subjects with multiple tooth agenesis? Eur J Orthod. 2008; 30: 249-253.
- Kenrad J, Vedtofte H, Andreasen JO, Kvetny MJ, Kjær I. A retrospective overview of treatment choice and outcome in 126 cases with arrested eruption of mandibular second molars. Clin Oral Invest. 2011; 15: 81- 87.
- Kjær I. Phenotypic classification of 90 dentitions with arrested eruption of first permanent mandibular maxillary molars. Sem In Orthod. 2010; 16: 172-179.
- Svanholt P, Svanholt M, Kjær I. Vertical movements and rotations of the ectopic mandibular canine in cross sectional and longitudinal studies on orthopantomograms from 54 patients diagnosed with mandibular canine ectopia. Dent. Hypotheses. 2020; 11: 52.
- Svanholt P, Svanholt M, Thomsen J, Kjær I. The ectopic mandibular canines can start tooth formation in three different locations. A case series study based on single orthopantomograms from 47 individuals. Eur Archives Ped Dent. 2024; 25: 191-199.
- Begtrup A, Grønastød H, Christensen IJ, Kjær I. Predicting lower third molar eruption on panoramic radiographs after cephalometric comparison of profile and panoramic radiographs. Online 2012. Eur J Orthod. 2013; 35: 460-466.
- Nodal M, Kjær I, Solow B. Craniofacial morphology in patients with multiple congenitally missing permanent teeth. Eur J Orthod. 1994; 16: 104-109.
- Riis LC, Kjær I, Mølsted K. Dental anomalies in different cleft groups related to neural crest developmental fields contributes to the understanding of cleft aetiology. J Plast Surg Hand Surg. 2014; 48: 126-131.
- Lauesen SR, Daugaard-Jensen J, Lauridsen E, Kjær l. Localised scleroderma en coup de sabre affecting the skin, dentition, and bone tissue within craniofacial neural crest fields: Clinical and radiographic study of six patients. Eur Arch Pediatr Dent. 2019; 20: 339-350.
- Bang E, Kjær I, Christensen LR. Etiologic aspects and orthodontic treatment of unilateral localized arrested tooth development combined with hearing loss. Am J Orthod Dentofac Orthop. 1995; 108: 154-161.
- Kjær I. Orthodontics and foetal pathology: a personal view on craniofacial patterning. Eur J Orthod. 2010; 32: 140-147.
- Kjær I. Can the reduced level of alveolar bone in the initial stages of juvenile periodontitis anterior to the first molar be explained as arrest in alveolar bone growth? Dental Hypotheses. 2013; 4: 44-49.
- Kjær I. Etiology-based diagnostics for improvement of treatment. Dental Hypotheses. 2016; 7: 31-33.
- Kjær I, Strøm C, Worsaae N. Regional aggressive root resorption caused by neuronal virus infection. Case Rep Dent. 2012; 2012:693240.
- Kjær I. Neuro-Orthodontics –a sub-speciality in orthodontics, important for diagnostics and treatment planning. E.C. Dent Sci. 2020; 10: 233-249.