Topographical Anatomy of the Tragus, Antitragus and Lower Concha in Near-Term Human Fetuses
- 1. Department of Otolaryngology-Head and Neck Surgery, Tohoku University Graduate School of Medicine, Japan
- 2. Department of Anatomy and Human Embryology, Complutense University, Spain
- 3. Department of Histology and Developmental Biology, Tokyo Dental College, Japan
- 4. Division of Internal Medicine, Iwamizawa Asuka Hospital, Japan
Abstract
Background and aim: The auricular cartilage plate contains multiple combinations of the fold and cave. We aimed to examine when and how the lower
part of the concha (lower concha) communicated with the orifice of the external acoustic meatus (EAM) since this point was still unclear in our previous studies.
Methods: We examined histological sagittal sections from 15 late-stage human fetuses (gestational age approximately 25-40 weeks; CRL 200-334 mm) as well as 8 midterm fetuses (11-16 weeks; CRL 60-125 mm).
Results: At midterm, the concha was absent below the crus helix and a communication between the tragus and EAM cartilages was developing. At near- term, although two specimens showed a delayed growth of helix, the tragus consistently continued to the upper, medial and/or posterior part of the EAM cartilages. The helix tail and antitragus were bar-like protrusions of the cartilage plate, respectively. Not only a framework cartilage of the lower concha but also the cartilaginous tragus and antitragus were often embedded in the subcutaneous tissue without external surface caves and grooves. Only three of 15 specimens carried a skin groove toward the concha loop.
Conclusion: Growth of the external skin feature was much delayed when compared with the internal cartilage growth. In the external or lateral view at birth, a cave below the crus might not correspond to the final lower concha but simply the EAM orifice. A tragus connecting to the antitragus in fetuses seemed not controversial to the classical theory that they are derived from the different pharyngeal arches.
Keywords
- Auricular cartilage plate
- Concha
- Tragus
- External acoustic meatus
- Pharyngeal arch
- Human fetus
Citation
Honkura Y, Verdugo-López S, Rodríguez-Vázquez JF, Kitamura K, Hirano-Kawamoto A, et al. (2024) Topographical Anatomy of the Tragus, Antitragus and Lower Concha in Near-Term Human Fetuses. Ann Otolaryngol Rhinol 11(3): 1339.
ABBREVIATION
ATM: Antitragus Muscle; EAM: External Acoustic Meatus; EAMC: External Acoustic Meatus Cartilages; IN: Incus; MA: Malleus; PAM: Posterior Auricular Muscle; SAM: Superior Auricular Muscle; PG: Parotid Gland; SCM: Sternocleidomastoideus Muscle; SQ: Squamosa of the Temporal Bone; TM: Temporalis Muscle
INTRODUCTION
Six hillocks (small tubercles of the surface ectoderm) are considered to develop into auricular cartilages [1-7].
Table 1: Six hillocks and each part of the final auricle
|
hillock-1 developing into |
tragus |
|
hillock-2 |
crus helix |
|
hillock-3 |
ascending helix |
|
hillocks-4 and 5 |
helix, scapha and antihelix |
|
hillock-6 |
helix tail and antitragus |
hillocks-1, 2 and 3 are the first pharyngeal arch derivatives.
hillocks-4, 5 and 6 are the second pharyngeal arch derivatives
According to the six hillocks theory [Table 1], the first pharyngeal groove separates the hillocks 1-3 from the hillocks 4-6 and, thus, the final intertragic groove (an incisura between the tragus and antitragus) corresponds to the initial pharyngeal groove. A belt-like mesenchymal condensation seems to be hypothesized to connect from the tragus, via the helix with crus helix, to the antitragus. Below, the crus helix is simply called the “crus”.
Honkura et al. [8], and Hashimoto et al. [9], might be limited studies showing an intermediate morphology of the growing auricular cartilage to connect the six hillocks with the final auricle. According to their observations, 1) the auricular cartilage makes a single, wavy cartilage plate when it appears; 2) the crus is discriminated by insertions of the superior and posterior auricular muscles from the other cartilage folds; 3) the antitragus muscle extends between the helix tail and antitragus, 4) in the single cartilage plate, the crus is located immediately above the tragus and, 5) the helix tail and antitragus are bar-like protrusions of the cartilage plate, respectively [Figure 1].
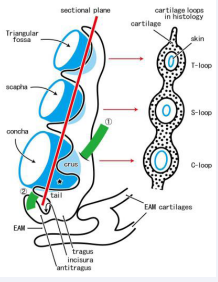
Figure 1 A correspondence between the three-dimensional hollow and fold of the growing auricular cartilage and their histology.
In near-term human fetuses, the auricular cartilage plate provides 3 or more funnels or hollows (colored blue) and, in the two-dimensional histology, theses morphologies are identified as multiple loops (T- or triangular loop; S- or scapha loop; C- or concha loop). The concha below the crus (star) is tentatively called “lower concha”. The growing crus is discriminated from the other cartilage folds according to an insertion of the superior auricular muscle (colored green;?). Likewise, the antitragus muscle (colored green; ?) extends between the antitragus and helix tail. The present study focuses topographical relation, including the continuation or separation, among the lower concha, antitragus, tragus, cartilages of the external acoustic meatus (EAM).
In two-dimensional histology, folding and grooving of the cartilage plate are often identified as “loops” of cartilage and the triangular, scapha and concha loops are seen [Figure 1]. In the aforementioned issues, insertion sites of the extrinsic auricular muscles are likely to be different from those in adults.
The concha is the largest hollow or fossa in the auricle in adults. However, still now, we did not know Hashimoto et al. [9] sometimes failed to find the concha loop of the cartilage, corresponding to the concha. Did the absence of loop suggest a mechanism to provide a large hollow other than the cartilage looping? Was the absence simply a result of an artifact by the sectioning process or sectional plane? Honkura et al. [8] and Hashimoto et al. [9] did not clearly describe the topographical relation among the tragus, antitragus and cartilages of the external acoustic meatus (EAM). Fetal morphologies of the tragus and antitragus seem to be quite important elements to evaluate anomalies. Consequently, the aim of this study was to revisit the growing lower part of the auricular cartilage plate especially its connection to the EAM cartilag
MATERIALS AND METHODS
The study was performed in accordance with the provisions of the Declaration of Helsinki 1995 (as revised in 2013). We used histological sections of the auricle from 15 late-stage human fetuses (gestational age approximately 25-40 weeks;
CRL 200-334 mm) as well as 8 midterm fetuses (gestational age approximately 11-16 weeks; CRL 60-125 mm).
The paraffin-embedded serial sections from 8 midterm fetuses were part of the large collection kept at the Department of Anatomy of the Universidad Complutense, Madrid, and the resulting embryos were obtained from miscarriages and ectopic pregnancies from the Department of Obstetrics of the University. The sectional planes were sagittal (2 specimens) or frontal [6]. The sections had been stained with hematoxylin and eosin (HE), azan. This study was approved by the Ethics Committees of Complutense University (B08/374). Understanding of the entire shape of the auricle is easier in frontal sections than sagittal sections at small fetuses (i.e., midterm fetuses), but sagittal sections are better in large fetuses such as the present 15 late- stage fetuses.
The 15 late-stage fetuses were part of the collection kept at the Department of Anatomy, Akita University, Akita, Japan. They had been donated by their families to the Department in 1975–1985 and preserved in 10% w/w neutral formalin solution for more than 30 years. Data for these specimens included the date of donation and the number of gestational weeks, but did not include the name of the family, obstetrician or hospital, or the reason for abortion. The use of these specimens for research was approved by the Akita University Ethics Committee (No. 1428) and the sixth author (G.M.) is one of members of the research project. Before routine procedures for paraffin embedding, the fetal specimens were decalcified by incubating them at room temperature in Plank-Rychlo solution (AlCl2/6H2O, 7.0 w/v%; HCl, 3.6; HCOOH, 4.6) for 3-7 days. The sections (0.1- or 0.2-mm interval) had been previously prepared by our group for studies of the nose, palate and tongue [10-12] as well as the auricle [8,9]. The sectional planes were sagittal (12 specimens) or horizontal (3) and the sections had been stained with HE. All photographs were taken with a Nikon Eclipse 80.
RESULTS
Observations of sections from midterm fetuses
A wavy cartilage plate was always seen in the anterosuperior side of the external acoustic meatus (EAM) but it did not contain any loops [Figure 2].
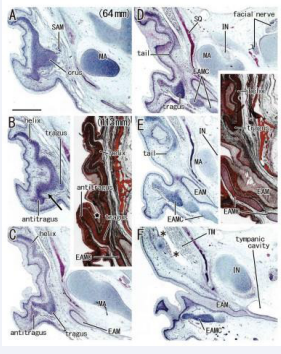
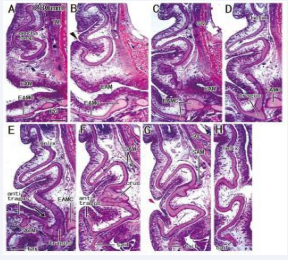
Figure 2: A simple cartilage plate in the early stage of auricle. Frontal sections. Panels A-F (HE staining), a fetus with 64 mm CRL; Two inserts (azan staining), a fetus with 113 mm. Panel A displays a plane 1.2 mm anterior to panel F. The superior auricular muscle (SAM) inserts to the crus (panel A). The tragus continues to cartilages of EAM (EAMC) in panels D and E. A candidate incisura (arrow in panel B), opening to the inferior side, is located almost 1.0 mm anterior to the EAM orifice (panel F). In inserts, a skin groove (star) does not correspond to the incisura because it does not open to the EAM. Asterisks indicate tissue damage during histological procedure. All panels and inserts were prepared at the same magnification (scale bar in panel A, 1 mm).
The crus was identified as a thickened part or thick tubercle of the cartilage plate according to extrinsic muscle insertions [Figure 2A]. The helix tail and antitragus were usually identified as protrusions at the lower end of the cartilage plate. The tragus was immediately below the crus and it continued to the superoanterior part of the EAM cartilage. In three of 10 midterm specimens, the incisura between the tragus and antitragus appeared absent [Figure 2] since, in them, a skin groove between or along the tragus and antitragus did not open or face to the EAM.
Observations of sections from late-stage fetuses
Figures are arranged from a small specimen to a large specimen although the size and thickness of auricular cartilages did not always correlate with the body size. The concha loop was usually (13/15) seen in the cartilage plate in the inferior or medial side of the crus [Figure 3, 5-7] and, in the superior side of the crus, no cartilage loop corresponded to or suggested the future concha.
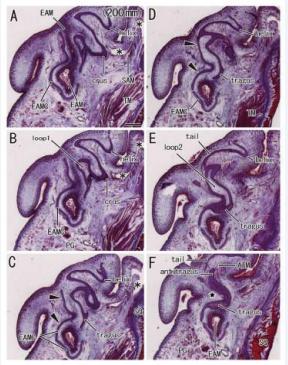
Figure 3: Double cartilage loops corresponding to the future lower concha in a fetus with 200 mm CRL. Sagittal sections. HE staining. Panel A (or Panel F) displays the most lateral (or medial) plane. Two cartilage loops (loop1 in panel B and loop2 in panel E) are seen in the cartilage plate between the crus (panels A and B) and tragus (panels C and E). No skin groove is seen growing toward the loops. Cartilages of EAM (EAMC), at the superolateral part (arrowheads in panels C and D), continues to the helix. A loose tissue sandwiched by the tragus and antitragus (star in panel F) does not correspond to the incisura because it does not direct toward the EAM. The antitragus accompanies the antitragus muscle (ATM in panel F). Asterisks indicate tissue damage during histological procedure. All panels were prepared at the same magnification (scale bar in panel A, 1 mm)
A single specimen carried double loops [Figure 3]. In the double loops, a lateral loop was connected to the EAM cartilage [Figure 3CD] and, in the immediately inferior side of the helix tail, a medial loop was seen connecting to the tragus and antitragus [Figure 3EF]. The tragus was also connected to the EAM cartilage.
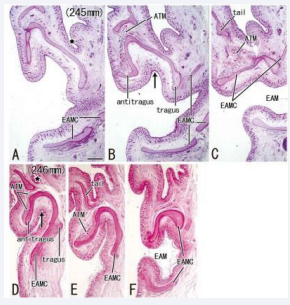
The concha loop was absent in two specimens with a deep skin groove toward the tragus (CRL 245 mm and 246 mm).
Figure 4: A poorly-developed helix without the concha loop in two fetuses (CRL, 245 and 246 mm). Sagittal sections. HE staining. Panels A and D (or Panels C and F) display the most lateral (or medial) plane in each of the specimens. The helix is thin and short and there is no concha loop. A deep skin groove separates the future auricle from the head temporal region (star in panels A and D) in spite of the small upper part. The incisura directs inferior (arrow in panels B and D) and opens to the EAM. All panels were prepared at the same magnification (scale bar in panel A, 1 mm).
In these two specimens [Figure 4], being independent of a delayed growth of the upper half of the auricular cartilage (i.e., the scapha and helix including the crus), the tragus, antitragus and EAM cartilages appeared to grow to a normal size at the stage of fetuses.
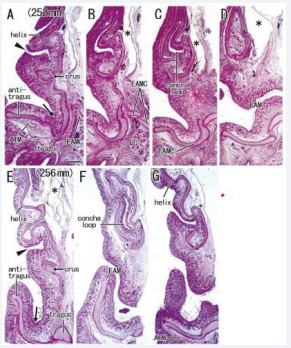
Figure 5: A continuation from the lower concha to the external acoustic meatus in the late stage of auricle. Sagittal sections. HE staining. The specimens had been removed from the head before histological procedure. Panels A-D, a fetus with 252 mm CRL; panels E-G, a fetus with 256 mm. Panels A and E displays the most lateral plane in each of the specimens. A candidate incisura (arrow in panels A and E) is seen near the tragus. The antitragus accompanies the antitragus muscle (ATM in panels A and G). In the medial or internal side of the crus, there is a cartilage loop corresponding to the future lower concha (panels C and E). A skin groove (arrowhead in panels A and E) inserts into the cartilage loop. Asterisks indicate tissue damage during histological procedure All panels were prepared at the same magnification (scale bar in panel A, 1 mm).
Figure 6: Helix tail extending inferolaterally to the tragus and external acoustic meatus cartilage in a fetus with 280 mm CRL. Sagittal sections. HE staining. Panel A (or Panel H) displays the most medial (or lateral) plane. A cartilage loop, corresponding to the future lower concha (panel A), is located medial side of the crus (panel F). A skin groove (arrowhead in panel B) is growing toward the cartilage loop. A candidate incisura (arrow in panel E) is seen near the tragus. The antitragus accompanies the antitragus muscle (ATM in panel E). The superior auricular muscle inserts to the crus (panel F). The tragus continues to the lower part of cartilages of EAM (EAMC) in panels C-E. Asterisks indicate tissue damage during histological procedure All panels were prepared at the same magnification (scale bar in panel A, 1 mm).
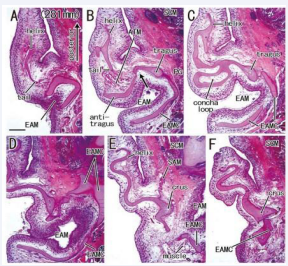
Figure 7: No skin groove growing toward a well-developed concha loop in a fetus with 281 mm CRL. Horizontal sections. HE staining. Panel A (or Panel F) displays the most inferior (or superior) plane. A candidate incisura (arrow in panel B) is seen between the tragus and antitragus. The antitragus accompanies the antitragus muscle (ATM) and the tragus continues to the cartilages of EAM (EAMC; panel B). A cartilage loop, corresponding to the future lower concha (panel C), is located 1.0-1.5 mm inferior side of the crus (panels E and F). The superior auricular muscle inserts to the crus (SAM; panel E). All panels were prepared at the same magnification (scale bar in panel A, 1 mm).
Below the crus, the concha loop often existed in the superior side of (even distant from) the EAM [Figure 5B, 6A and 7C]. Notably, the majority (nine specimens) did not carry a skin groove or cave corresponding to the future lower concha [Figure 3BE and 7C]. Conversely, in the other three specimens [Figure 5AE and 6B], a skin grove approached the bottom of the concha loop [Figure 5CF]. However, the skin groove did not open to or connect with a large cave of the EAM orifice. In all 15 near-term specimens including two specimens with a poorly developed helix, the tragus continued to the upper, medial and/or posterior part of the EAM cartilages. The helix tail rarely extended inferolaterally to the EAM and its cartilages at near-term [Figure 6E-G].
The intertragic incisura, one of prominent features in the auricle, exhibited a variation: the skin groove opened superiorly [Figure 5AE and 6E] or inferiorly [Figure 4BD and 7B] to the EAM. When the antitragus was located below (or above) the EAM orifice, the incisura opened superiorly (or inferiorly). The groove appeared to be “budding” from the EAM [Figure 7BC]. In five specimens larger than 252 mm CRL [Figure 5-7], a skin groove inserted into the incisura, but an incisura opening upward was limited [Figure 5AE and 6E].
Consequently, the concha loop was a limited structure corresponding to the future concha, but it was not positioned for the future entire concha but for the lower concha below the crus. Even at near-term, not only a cartilaginous framework of the lower concha but also the cartilaginous tragus, antitragus and intertragic incisura were often embedded in the subcutaneous tissue without external skin grooves and folds accompanied.
DISCUSSION
To provide a final auricular morphology, cartilage loops (a cave or hollow in 3-dimension; Figure 1) should be lined by the skin. However, even at near-term, no skin grove usually inserted into the concha loop. In limited three specimens, a skin groove was seen corresponding to the future lower concha, but it did not widely open to and continue to a large cave at the EAM orifice. Moreover, the cartilage framework “concha loop” was located below the crus. Thus, it seemed not to correspond to the future entire concha but the lower concha. Therefore, conversely, a large fossa “concha” might appear after birth. Likewise, usually, the intertragic incisura did not obtain the final topographical relation with the EAM orifice. Often, the incisura did not open superiorly but inferiorly toward the EAM orifice. After birth, the lower concha, tragus, antitragus and intertragic incisura would become established as structures covered by skin and they would obtain the final arrangement around the EAM orifice. Pediatric ENT doctors touch and find “cartilages” around a concha-like fossain newborns and even in premature babies, but the framework may not correspond to the real auricular cartilage plate but EAM cartilages.
Hashimoto et al. [9], demonstrated a lateral view of the auricle from a fetus with 230 mm CRL in their Figure 1: the auricle appeared to carry two large caves (their-termed “concha”) above and below the crus. The size of the fetuses was almost same as the present late-stage specimens. Actually, their concha was similar to the adult morphology, but the intertragic incisura was very close to the EAM orifice. According to the present observations, a cave or hollow below the crus at near-term was most likely to correspond to the EAM orifice itself, not the real concha. A framework cartilage of the lower concha (i.e., the concha loop) were still embedded in the subcutaneous tissue at near-term.
The tragus and antitragus in fetuses were not separated but continuous: these structures were the medial and lateral ends of a short cartilage bar, respectively. This morphology evoked a question to the classical dichotomy: the first pharyngeal archderived tragus and the second arch-derived antitragus [Table 1]. The EAM corresponds to the first pharyngeal groove or cleft between the first and second pharyngeal arches. Thus, people may consider that the second arch-derived antitragus should be connected to the lower wall cartilage of EAM. However, the antitragus was connected to the EAM cartilage “via the tragus”. Actually, it seemed difficult, or even nonsense, to discriminate the upper wall element from the lower element of EAM cartilages because the growing EAM cartilages are composed of multiple longitudinal belt-like cartilages connecting each other [14]. The first and second arch-derived mesenchymal tissues together seem to provide the EAM cartilages. Likewise, the tragus and antitragus were likely to be of a mixed or dual origin.
The concha loop as well as most parts of the helix was absent in the present two specimens [Figure 4]. Notably, being independent of a delayed growth of the upper half of the auricular cartilage, the tragus, antitragus and EAM cartilages were well developed. Rather the classical dichotomy in which the tragus develops independent of the antitragus, the upper and lower auricular cartilages might develop under different systems of induction, respectively.
Finding Source
This study was supported by Grant-in-Aid for Scientific Research (No.18K16826 for Yohei Honkura) from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
REFERENCES
- Streeter GL. Development of the auricle in the human embryo. Carnegie Institution of Washington. Contrib Embryol. 1922; 14: 111- 138.
- Wood-Jones F, Wen IC. The development of the external ear. J Anat. 1934; 68: 525-533.
- Siegert R, Weerda H, Remmert S. Embryology and surgical anatomy of the auricle. Facial Plast Surg. 1994; 10: 232-243.
- Karmody CS, Annino DJ Jr. Embryology and anomalies of the external ear. Facial Plast Surg. 1995; 11: 251-256.
- Park C, Roh TS. Congenital upper auricular detachment. Plast Reconstr Surg. 1999; 10: 488-490.
- Porter CJW, Tan ST. Congenital auricular anomalies: topographic anatomy, embryology, classification, and treatment strategies. Plast Reconstr Surg. 2005; 115: 1701-1712.
- Moneta LB, Quintanilla-Dieck L. Embryology and anatomy of the ear. Operative Techniques Otrolaryngol. 2017; 28: 66-71.
- Honkura Y, Hayashi S, Kim JH, Murakami G, Abe H, Rodríguez- Vázquez JF, et al. Development and growth of auricular cartilages and muscles: a study using human fetuses. Int J Pediatr Otorhinolaryngol. 2020; 133: 109973.
- Hashimoto C, Kitamura K, Yamamoto M, Honkura Y, Murakami G, Abe H, et al. Auricular cartilage configuration: a histological study using late-stage human fetuses and adult cadavers. Anat Rec. 2021; 304: 2661-2672.
- Kim JH, Oka K, Jin ZW, Murakami G, Rodríguez-Vázquez JF, Ahn SW, et al. Fetal development of the incisive canal, especially of the delayed closure due to the nasopalatine duct: a study using serial sections of human fetuses. Anat Rec. 2017; 300: 1093-1103.
- Kim JH, Shibata S, Abe H, Murakami G, Rodríguez-Vázquez JF. Topographical variations of the incisive canal and nasopalatine duct in human fetuses. Anat Cell Biol. 2019; 52: 426-435.
- Yamamoto M, Hayashi H, Honkura Y, Hirano-Kawamoto A, Katori Y, Murakami G, et al. Nasal capsule ossification: a histological study using human fetuses to connect between morphologies of the fetus and adult. J Anat. 2023; 243: 517-533.
- Yamamoto M, Hirota Y, Watanabe G, Taniguchi S, Murakami G, Rodriguez-Vazquez JF, Abe S. Development and growth of median structures of the human tongue: i histological study using human fetuses and adult cadavers. Anat Rec. 2024; 307: 426-441.
- Ikari Y, Katori Y, Ohtsuka A, Rodríguez-Vázquez JF, Abe H, Kawase T, et al. Fetal development and variations in the cartilages surrounding the human external acoustic meatus. Ann Anat. 2013; 195: 128-136.e.