Evaluation of Cystic Fibrosis Medication Adherence and Barriers in 2020 and 2022
- 1. Froedtert Health, 9200 W Wisconsin Ave, Milwaukee, WI 53226, USA
- 2. Concordia University of Wisconsin School of Pharmacy, 12800 N Lakeshore Drive, Mequon, WI 53097, USA
- 3. Medical College of Wisconsin School of Pharmacy, 8701 Watertown Plank Rd, Milwaukee, WI 53226, USA
Abstract
Background: Individuals with Cystic Fibrosis (CF) tend to struggle with adherence due to complex medication regimens comprised of multiple daily dosing and various routes of administration [1]. A Medication Possession Ratio (MPR) can be calculated from refill history to provide an objective measure of overall adherence. This investigation intends to assess changes in medication adherence rates, as measured by MPR, during two distinct timeframes that encompass the introduction of Elexacaftor/Tezacaftor/Ivacaftor (ETI) use for this population.
Methods: Investigators retrospectively gathered patient characteristics and fill histories for patients diagnosed with CF, on medications evaluated in the project, and seen by the adult CF team. A MPR of ≥ 80% was considered adherent. Data was summarized using descriptive statistics. Adherent and less- adherent patients were compared using the Chi-square test for categorical variables and the Wilcoxon rank sum test for continuous variables.
Results: The 2022 assessment period mean composite MPR was 72% (± 23%) compared to 69% (± 25%) in 2020 (p = 0.533). Of the 89 patients in 2022 assessment period, 89% (79/89) were on a CFTR modulator compared to 85% (76) in 2020, 49% (44) were on azithromycin compared to 52% (46) in 2020, 73% (65) were on dornase alpha compared to 92% (82) in 2020, and 48% (43) were on inhaled antibiotics compared to 67% (60) in 2020.
Conclusions: When using composite MPR to assess patients’ adherence to their entire treatment regimen, calculations do not reveal a statistically significant change in adherence despite less patients prescribed CF other non-modulator maintenance therapies two-years after initiating ETI.
Keywords
Medication; Adherence; Outpatient; Clinic; Pharmacist; Pharmacy; Barrier
Citation
Belmonte L, Fleischman M, Hinz R, Wendland E (2024) Evaluation of Cystic Fibrosis Medication Adherence and Barriers in 2020 and 2022. Clin Res Pulmonol 10(1): 1068.
BACKGROUND
Individuals with Cystic Fibrosis (CF) tend to struggle with adherence due to complex medication regimens [1]. Daily treatment regimens can include multiple daily dosing and various routes of administration (oral liquid, oral pills, inhalers, nebulized, inter-nasal, subcutaneous injections, etc.). Survey respondents with CF reported significant time burdens associated with treatments and were on a median of 7 daily therapies [2].
There are many barriers that may impact a patient’s adherence including access to medications, system hindrances and patient understanding. Public insurance coverage has shown to be associated with lower maximum Percent Predicted Forced Expiratory Volume over 1 Second (max ppFEV1) at age six [3]. 5 Patients may suffer from depression, have difficulties with time management, or have perceived doubts about the necessity of treatments [4]. Poor adherence to airway maintenance therapies and inhaled antibiotics can lead to increased risk of exacerbations, weight loss and diminished lung function, which can increase heath care costs and lead to faster disease progression [1-6].
Monitoring adherence through medication refill tracking, by using Medication Possession Ratio (MPR), provides an objective measure for clinicians to gauge overall adherence. MPR has been utilized in the medical literature. One study that included patients with CF at least one prescription fill for a CF pulmonary medication found an association with low MPR and higher acute care costs [6].
In the 2020 assessment period, MPR was used to evaluate medication adherence in patients with CF at Froedtert and the Medical College of Wisconsin (F&MCW) [7]. Since that assessment, more patients at the center have transitioned to the highly effective CFTR modulator, Elexacaftor-Tezacaftor-Ivacaftor (ETI). Patients on ETI have demonstrated substantial improvements in pulmonary function, reductions in exacerbations and patient- reported symptoms [8,9]. It is expected that approximately 90% of the CF population is eligible to be treated with ETI based on having one copy of the F508del-CFTR mutation [10]. With clinical improvements after starting ETI therapy, many patients may have elected to be less adherent or stop other medications classes. There is ongoing investigation supported by the CF Foundation to evaluate if the withdrawal of some burdensome CF therapies has clinical implications.
Froedtert & the Medical College of Wisconsin’s (F&MCW) Pulmonary Clinic aims to improve adherence in the CF population to help promote better overall health and reduce hospitalizations. Investigators intended to assess how medication adherence rates have changed since the previous assessment and identify factors that are associated with medication adherence. Results from this evaluation are important for the CF clinic’s ongoing continuous quality improvement initiatives to improve patients’ medication adherence and will add to the current understanding of patient factors that may lead to changes in medication adherence.
METHODS
This project occurred at a single institution and consisted of a cross-sectional evaluation and a retrospective chart review. The F&MCW Pharmacy Research Committee deemed this project as quality improvement and it was classified as exempt by the Medical College of Wisconsin Institutional Review Board. Independent collection of patient characteristics and medication refill history was completed by four investigators who went through training to increase consistency of data gathered. The investigators cross-checked fill histories and data abstracted from the chart review for accuracy. Patients included in this assessment met the following criteria: (a) ≥ 18 years of age, (b) had a diagnosis of CF, (c) were on at least one of the medications evaluated in the assessment (azithromycin, dornase alpha, inhaled antibiotic [tobramycin, aztreonam, ceftazidime, and colistin], and/or CFTR modulator [elexacaftor/tezacaftor/ ivacaftor, tezacaftor/ivacaftor, ivacaftor]), and (d) were followed by the adult CF team prior to 12/31/21. For each patient meeting these criteria, fill history data was collected from 4/1/2021 to 4/30/2022 and patient characteristic and barriers information was collected up until January 2023. Outcomes and data were then compared to outcomes and data from an earlier time frame of 4/1/2019 through January 2021, which were collected in a similar manner [7]. These timeframes are henceforth referred to as the 2022 assessment period and 2020 assessment period, respectively.
Data Collection
Data for generating the MPR calculation came from electronic medical record refill histories, reports from specialty pharmacies with a CF liaison, and reports from medication manufacturers. For patients with incomplete refill history despite the mentioned methods, investigators then also verified available refill history with SureScripts® and/or called all presumed filling pharmacies to obtain refill dates for the pertinent CF medications. Additionally, MPR calculations accounted for patient hospitalizations and IV antibiotics.
Data pertaining to patient characteristics were gathered from the electronic medical record and PortCF, the national CF registry with data on patients whom provide consent to participate in data collection. The perception of health rating came from a question used in the CF-Questionnaire Revised (QR). Ultimately, the data collected was used to describe patient characteristics for those considered adherent based on a composite MPR of ≥ 80%. For patients with an MPR < 50% in any medication category, the investigational team shared the patient’s MPR calculation results for their medications evaluated and interviewed the patient to assess for barriers in taking CF medications.
Statistical Analysis
Data was summarized using descriptive statistics. Counts and percentages were used to summarize categorical variables. Median and range along with the mean and standard deviation were used for continuous variables. A Chi-square test was used to compare categorical variables between patients who were adherent to the prescribed medication and those who were not; unless there were less than 5 individuals in a group, then Fisher’s Exact test was utilized. Wilcoxon rank sum test was used for comparing continuous variables. A p-value < 0.05 was considered statistically significant.
RESULTS
Within the 2022 assessment period, 46% (47/103) had an MPR ≥ 80%. Of those with an MPR ≥ 80%, the mean age was 36.9 ± 13.1 (p = 0.017), 60% were male (28/47, p = 0.092), mean FEV1 was 77.9 ± 26.0 (n = 46/47, p = 0.758), mean number of pharmacies 2.3 ± 0.8, (p = 0.624), 68% had commercial insurance (n = 32/47, p = 0.557), and 54% perceived their health a good (n = 25/47, p = 0.853) (Table 1).
Table 1: Patient characteristics within both assessment timeframes.
|
Characteristics |
2020, N = 105 |
2022, N = 103 |
||
|
Non- Adherent |
Adherent |
Non- Adherent |
Adherent |
|
|
N = 62 |
N = 43 |
N = 56 |
N = 47 |
|
|
Age (Mean ± SD) |
31.5 ± 9.8* |
38.4 ± 12.9* |
31.0 ± 10.3† |
36.9 ± 13.1† |
|
Sex |
||||
|
Male |
32 (51.6) |
25 (58.1) |
27 (48.2) |
28 (59.6) |
|
Female |
30 (48.4) |
18 (41.9) |
29 (51.8) |
17 (36.2) |
|
Q |
0 |
0 |
0 |
2 (4.3) |
|
FEV1 (Mean ± SD) |
71.8 ± 25.2 |
71.7 ± 26.3 |
75.8 ± 26.7 |
77.9 ± 26.0 |
|
No value |
3 |
0 |
2 |
1 |
|
Number of Filling Pharmacies (Mean ± SD) |
2.5 ±1.2 |
2.5 ± 1.3 |
2.3 ± 0.7 |
2.3 ± 0.8 |
|
Primary Insurance |
||||
|
Commercial |
49 (79.0) |
32 (74.4) |
36 (64.3) |
32 (68.1) |
|
Medicaid |
7 (11.3) |
4 (9.3) |
6 (10.7) |
7 (14.9) |
|
Medicare |
6 (9.7) |
7 (16.3) |
14 (25.0) |
8 (17.0) |
|
Perception of Health |
||||
|
Excellent |
- |
- |
13 (24.1) |
11 (23.9) |
|
Good |
- |
- |
28 (51.9) |
25 (54.3) |
|
Fair |
- |
- |
11 (20.4) |
7 (15.2) |
|
Poor/Very poor |
- |
- |
2 (3.7) |
3 (6.5) |
|
No Value |
- |
- |
2 |
1 |
*p = 0.006; †p = 0.017; SD = Standard Deviation; Q: Queer; FEV1: Forced Expiratory Volume over 1 Second
Data Comparison between Assessment Periods
Among the 103 patients evaluated in the 2022 assessment period, 89 of those patients also had data in the 2020 assessment period. Of those 89 patients in the 2022 assessment period, 89% (79/89) were on a CFTR modulator compared to 85% (76) in 2020 (p = 0.5), 49% (44) were on azithromycin compared to 52% (46) in 2020 (p = 0.8), 73% (65) were on dornase alpha compared to 92% (82) in 2020 (p < 0.001), and 48% (43) were on inhaled antibiotics compared to 67% (60) in 2020 (p < 0.01).
Table 2: MPR Calculations for 2020 and 2022 assessment periods.
|
|
All patients |
Patients in both time frames (N = 89) |
||
|
2020 (N = 105) |
2022 (N = 103) |
2020 |
2022 |
|
|
Composite MPR |
||||
|
Median (min - max) |
0.74 (0.00 -1.00) |
0.78 (0.00 - 1.00) |
0.74 (0.00 - 1.0) |
0.79 (0.00 - 1.0) |
|
Mean ± SD |
0.67 ± 0.28 |
0.70 ± 0.25 |
0.69 ± 0.25 |
0.72 ± 0.23 |
|
MPR modulators |
||||
|
Median (min - max) |
0.97 (0.00 -1.00) |
0.92 (0.21 - 1.00) |
0.96 (0.00 - 1.0) |
0.92 (0.43 - 1.0) |
|
Mean ± SD |
0.86 ± 0.25 |
0.88 ± 0.15 |
0.88 ± 0.20 |
0.89 ± 0.12 |
|
Not prescribed |
14 (13%) |
10 (10%) |
10 (11%) |
7 (8%) |
|
No MPR values* |
3 (3%) |
3 (3%) |
3 (3%) |
3 (3%) |
|
MPR Azithromycin |
||||
|
Median (min - max) |
0.78 (0.00 -1.01) |
0.84 (0.04 - 1.00) |
0.74 (0.00 - 1.0) |
0.84 (0.04 - 1.0) |
|
Mean ± SD |
0.70 ± 0.32 |
0.71 ± 0.31 |
0.69 ± 0.31 |
0.73 ± 0.29 |
|
Not prescribed |
47 (45%) |
55 (53%) |
43 (48%) |
45 (51%) |
|
MPR Dornase Alpha |
||||
|
Median (min - max) |
0.64 (0.00 -1.00) |
0.67 (0.00 - 1.00) |
0.64 (0.00 - 1.0) |
0.70 (0.00 - 1.0) |
|
Mean ± SD |
0.59 ± 0.34 |
0.55 ± 0.35 |
0.61 ± 0.32 |
0.56 ± 0.35 |
|
Not prescribed |
9 (9%) |
25 (24%) |
7 (8%) |
24 (27%) |
|
MPR Inhaled Antibiotics |
||||
|
Median (min - max) |
0.7 (0.0 - 1.0) |
0.7 (0.0 - 1.0) |
0.71 (0.00 - 1.0) |
0.73 (0.00 - 1.0) |
|
Mean ± SD |
0.6 ± 0.3 |
0.6 ± 0.3 |
0.63 ± 0.31 |
0.65 ± 0.31 |
|
Not prescribed |
33 (31%) |
55 (53%) |
29 (33%) |
46 (52%) |
ETI = Elexacaftor/Tezacaftor/Ivacaftor; MPR = Medication Possession Ratio; SD = standard deviation;
*Patients who were receiving ETI therapy from manufacturer extension study.
Table 2 displays the medication possession ratio calculated for these patients as well as the full study population evaluated in both assessment periods. For the 89 patients in each assessment period, the 2022 assessment period mean composite MPR was 72% (± 23%) compared to 69% (± 25%) in 2020 (p = 0.533) (Table 2).
When reviewing total number of patients in both assessment periods, irrespective of patients with data in both assessment periods, mean adherence by medication category remained relatively unchanged (Table 2). Overall, mean adherence to modulators was in the upper 80% while mean adherence to other non-modulator maintenance therapies was 70% for azithromycin, mid to upper 50% for dornase alpha, and 60% for inhaled antibiotics. Additionally, mean composite MPR was still slightly higher in the 2022 assessment period compared to the 2020 assessment period, but this difference remained non- significant (Table 2).
Barriers to Adherence
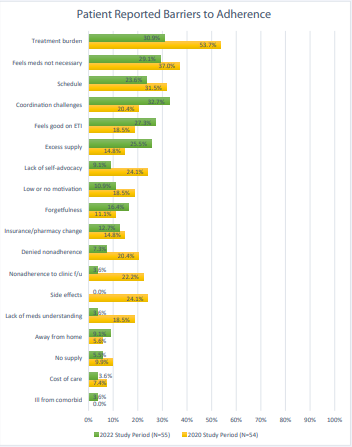
For patients with data in both assessment periods and a MPR < 50%, the top barriers to medication adherence identified in the 2022 assessment period included coordination challenges (18/55; 33%), treatment burden (17/55; 31%), patients feeling as though CF medications are not necessary (16/55; 29%), patients feeling good on modulator therapy (15/55; 27%), patients having excess supply of CF medication (14/55; 26%), and time management issues (13/55; 24%) (Figure 1).
Figure 1: Reasons for non-adherence within 2020 and 2022 assessment periods.
CF = Cystic Fibrosis; ETI = Elexacaftor/Tezacaftor/Ivacaftor; f/u = follow up
DISCUSSION
This analysis used retrospective data to evaluate relationships between CF medication adherence and patient variables. Based on patient characteristics in both assessment periods, only age was statistically significant for a difference in adherence, where patients defined as adherent (MPR > 80%) had a higher mean age than non-adherent patients. This finding is similar to results of a retrospective cohort analysis for patients with CF with ≥ 1 dornase alfa pharmacy claim where overall adherence began to increase in patients in their early to mid-thirties compared to a decline in adherence seen between the ages of 13-30 [11]. It can be hypothesized that older patients see the value in taking their medications if they have a worse prognosis or had a history of better disease management success with adherence.
It is important to note, however, that adherence is difficult to accurately capture as it depends on many factors including type of measure and medications [11,12]. In addition to this, our data may highlight that our patient population may have been healthier than the general CF population since the providers at F&MCW adult CF clinic agreed to stopping therapies in patients with high ppFEV1 values and no evidence of bronchiectasis. Despite a significantly lower number of patients taking inhaled treatments in the second time frame, the adherence to other non-modulator maintenance therapies remained < 80%, which suggests a continued need to optimize adherence for those maintained on other non-modulator maintenance therapies.
Based on our findings, a slightly higher portion of patients in the 2022 assessment period had modulators prescribed than in the 2020 assessment period while fewer were prescribed dornase alpha and inhaled antibiotics (i.e. tobramycin, aztreonam, ceftazidime, and colistin). At our institution, there may have been fewer patients prescribed non-modulator maintenance therapies over time as a means to simplify a patient’s regimen if patients and providers were in agreement that the patient’s lung function and overall health had been stable after having been on highly effective modulator therapy. Similarly, in the SIMPLIFY trial, results found that discontinuation of dornase alpha and hypertonic saline in certain patients with CF is non-inferior to continuation of such therapies with respect to short-term change in lung function [10]. The goal of the SIMPLIFY study was to better understand if it is safe to stop these often times burdensome CF airway clearance therapies as it is not known if these therapies further improve lung function over what is already gained by patients taking a highly effective CF modulator.
For patients with MPRs < 50%, the most common reported barriers were coordination challenges, treatment burden, and CF medications being perceived as unnecessary (Table 2). However, the number of patients that reported a barrier of “treatment burden” decreased between assessment periods as did the number who reported they perceived CF medications were unnecessary. This could be due to fewer people being on inhaled therapies as we saw in the MPR calculations, presented in (Table 2), patients feeling better on ETI therapy, or education they received in 2020 about therapies when discussing barriers. Additionally, the clinic (re)emphasized education about modulator efficacy in disease state management as well as safety monitoring needs with implementing workflows for follow-up which could have contributed to fewer reports of nonadherence to clinic visits as a listed barrier to adherence in the second time frame. One unexpected topic patients mentioned in the 2022 assessment period was their comfort with discussing adherence barriers with the treatment team after direct conversations about non-adherence were introduced in the 2020 assessment period. This finding may be reflected in the change observed with the barrier “denied nonadherence” and emphasizes the importance of CF treatment teams encouraging direct yet empathetic conversations with patients.
Lastly, this assessment is not without its limitations. Data collected retrospectively has inherent limitations as it is collected based on historical information requiring recollection, thus subject to recall bias, and accurate record keeping. There is also a risk for errors in transmission when multiple individuals collect and enter data (i.e. collecting fill records over the phone), which was necessary when a fill report could not be run for certain medications or pharmacies. However, the investigators implemented measures to mitigate these risks for inter-rater bias by training those who collected data, cross checking data that was collected and use of more accurate historical records based on what they learned from data collection in the first timeframe.
CONCLUSIONS
Based on findings within this assessment, patient characteristics that would suggest better or worse adherence to CF regimens were not identified except for the adherent populations having a significantly older mean age. A drastic decrease in number of patients on inhaled therapies was noted, which may explain changes in barriers to adherence, but this did not seem to impact composite adherence based on our MPR calculations. At this time, conclusions cannot be made on health outcomes as this assessment was not structured to do so. For this reason, there is still a need for literature to help CF researchers better understand what other non-modulator maintenance therapies are indicated in patients on highly effective modulators and further guidance on when such therapies can be discontinued.
ACKNOWLEDGEMENTS
We would like to thank Patricia Pfahler, BSN, RN and David Schmid, BSN, APNP for their help with data acquisition.
CONTRIBUTORS
Each author had full access to the data and takes responsibility for the integrity and accuracy of this study. All authors were responsible for acquisition of data and contributed to and approved of the final submitted manuscript. LB and MEF were responsible for the study design, data analysis and interpretation, and the writing of the manuscript.
ETHICS
This project was approved (PRO00043724) by the Pharmacy Research Committee with Froedtert and the Medical College of Wisconsin as a quality improvement project and not human subjects research it was classified as exempt by the Medical College of Wisconsin Institutional Review Board.
REFERENCES
- Dodd ME, Webb AK. Understanding non-compliance with treatment in adults with cystic fibrosis. J R Soc Med. 2000; 93: 2-8. PMID: 10911812; PMCID: PMC1305877.
- Sawicki GS, Sellers DE, Robinson WM. High treatment burden in adults with cystic fibrosis: challenges to disease self-management. J Cyst Fibros. 2009; 8: 91-96. doi: 10.1016/j.jcf.2008.09.007. Epub 2008 Oct 26. PMID: 18952504; PMCID: PMC2680350.
- Dickinson KM, Psoter KJ, Riekert KA, Collaco JM. Association between insurance variability and early lung function in children with cystic fibrosis. J Cyst Fibros. 2022; 21: 104-110. doi: 10.1016/j. jcf.2021.06.006. Epub 2021 Jun 24. PMID: 34175244; PMCID: PMC8695631.
- Bishay LC, Sawicki GS. Strategies to optimize treatment adherence in adolescent patients with cystic fibrosis. Adolesc Health Med Ther. 2016; 7: 117-124. doi: 10.2147/AHMT.S95637. PMID: 27799838; PMCID: PMC5085292.
- Eakin MN, Bilderback A, Boyle MP, Mogayzel PJ, Riekert KA. Longitudinal association between medication adherence and lung health in people with cystic fibrosis. J Cyst Fibros. 201; 10: 258-264. doi: 10.1016/j.jcf.2011.03.005. Epub 2011 Mar 31. PMID: 21458391; PMCID: PMC3114200.
- Quittner AL, Zhang J, Marynchenko M, Chopra PA, Signorovitch J, Yushkina Y, et al. Pulmonary medication adherence and health- care use in cystic fibrosis. Chest. 2014; 146: 142-151. doi: 10.1378/ chest.13-1926. PMID: 24480974.
- Fleischman M. 272: Patient factors based on adherence to cystic fibrosis medications. Journal of Cystic Fibrosis. 2021; 20: S131.
- Middleton PG, Mall MA, D?evínek P, Lands LC, McKone EF, Polineni D, et al; VX17-445-102 Study Group. Elexacaftor-Tezacaftor-Ivacaftor for Cystic Fibrosis with a Single Phe508del Allele. N Engl J Med. 2019; 38: 1809-1819. doi: 10.1056/NEJMoa1908639. Epub 2019 Oct 31. PMID: 31697873; PMCID: PMC7282384.
- Heijerman HGM, McKone EF, Downey DG, Van Braeckel E, Rowe SM, Tullis E, et al; VX17-445-103 Trial Group. Efficacy and safety of the elexacaftor plus tezacaftor plus ivacaftor combination regimen in people with cystic fibrosis homozygous for the F508del mutation: a double-blind, randomised, phase 3 trial. Lancet. 2019; 394: 1940- 1948. doi: 10.1016/S0140-6736(19)32597-8. Epub 2019 Oct 31. Erratum in: Lancet. 2020 May 30; 395(10238): 1694. doi: 10.1016/ S0140-6736(20)31021-7. PMID: 31679946; PMCID: PMC7571408.
- Mayer-Hamblett N, Ratjen F, Russell R, Donaldson SH, Riekert KA, Sawicki GS, et al ; SIMPLIFY Study Group. Discontinuation versus continuation of hypertonic saline or dornase alfa in modulator treated people with cystic fibrosis (SIMPLIFY): results from two parallel, multicentre, open-label, randomised, controlled, non-inferiority trials. Lancet Respir Med. 2023; 11: 329-340. doi: 10.1016/S2213- 2600(22)00434-9. Epub 2022 Nov 4. PMID: 36343646; PMCID: PMC10065895.
- Nasr SZ, Chou W, Villa KF, Chang E, Broder MS. Adherence to dornase alfa treatment among commercially insured patients with cystic fibrosis. J Med Econ. 2013; 16: 801-808. doi: 10.3111/13696998.2013.787427. Epub 2013 Apr 4. PMID: 23506540.
- Hoo ZH, Curley R, Walters SJ, Campbell MJ, Wildman MJ. Exploring the implications of different approaches to estimate centre-level adherence using objective adherence data in an adult cystic fibrosis centre - a retrospective observational study. J Cyst Fibros. 2020; 19: 162-167. doi: 10.1016/j.jcf.2019.10.008. Epub 2019 Oct 31. PMID: 31678011.