Inhibition of Inducible Nitric Oxide Synthase Reactivates Growth of Dormant Mycobacterium Tuberculosis within Infected Macrophages
- 1. Department of CSIR-National Chemical Laboratory, Organic Chemistry Division, India
- 2. Department of Academy of Scientific and Innovative Research (Asif), India
Abstract
Tuberculosis is one of the top ten causes of human death. The generation of reactive nitrogen intermediates within the macrophages and to some extent in Mycobacterium tuberculosis cells as well is well documented. Release of NO from lung macrophage cells after challenge with M. tuberculosis bacilli is reported. However, the link between these reactive nitrogen intermediates and M. tuberculosis survival inside macrophage is still far from resolved. In this study, we have shown that supplementation of medium with nitrite (10mM) transformed the actively growing intracellular M. tuberculosis cells into dormant form. This is confirmed from colony forming units, acid fast staining and drug resistance studies. However, exposure of infected macrophage cells with iNOS inhibitor (1400 W) at 5μM concentration resulted in two-fold increase of intracellular growth of mycobacterial cells within 8 days of infection. Similar trend is also observed on nitrite exposed intracellular M. tuberculosis cells. Whereas 1400W does not have any significant effect on extracellular survival of either active or dormant M. tuberculosis cells. Therefore, this report suggests that both host NO and mycobacterial NO together play important role to achieve and maintain dormancy during intracellular stage. In addition, the inhibition of host derived nitric oxide could help to redesign the tuberculosis combination therapy..
Keywords
• Intracellular Mycobacteria
• Dormancy
• iNOS Inhibitor
• Nitrite
• Nitric Oxide
CITATION
Agrawal S, Yeware A, Sarkar D (2024) Inhibition of Inducible Nitric Oxide Synthase Reactivates Growth of Dormant Mycobacterium Tuberculosis within Infected Macrophages. Clin Res Pulmonol 10(1): 1066.
INTRODUCTION
Mycobacterium tuberculosis (M. tuberculosis) is the causative agent of Tuberculosis (TB) discovered by Robert Koch in 1882. One-quarter of the population are infected with the latent form of M. tuberculosis [1]. The WHO has called for the end of TB by the year 2035 [2]. The major challenges of TB eradication are the increasing cases of drug-resistant bacilli, undiagnosed latent or dormant bacilli, and the length/cost of treatment [3]. M. tuberculosis infects lung macrophages, which serve as its primary host. Within the macrophage, M. tuberculosis can survive (as a latent or dormant form) or proliferate (active form) instead of being killed because of the capacity of M. tuberculosis cells to be modulated under oxidative stressed condition. Therefore, it is necessary to understand the role of redox system with greater detail on its survival inside alveolar macrophages.
Earlier reports have shown that macrophages exposed to M. tuberculosis bacilli induce NOS2 gene expression and increase NO production [4], suggesting that host-derived NO could positively influence the conversion of actively growing bacilli into the dormant stage during Mtb pathogenesis and survival. Although the role of host NO in murine macrophages is well established as a protective agent against M. tuberculosis, it is not the same in humans. In addition, it has been suggested that immune-activated mouse macrophages produce 1,000 times more NO than human macrophages because of their difference in iNOS characteristics [5]. Recently, nitrite has been found to act as an inducer of mycobacterial dormancy even under aerobic conditions [6]. The nitrite reductase (NO forming) enzyme (nirK) converts nitrite into NO to influence this conversion [7]. However, the extent to which the NO produced by arginine and nitrite respectively and influence the M. tuberculosis bacilli life within the host is yet to be determined.
Therefore, in this report, using the red fluorescent reporter M. tuberculosis bacilli through the High Content Imaging tool, we have shown that nitrite induces dormancy of intracellular M. tuberculosis bacilli. Furthermore, NO produced by iNOS of host macrophages and nitrite reductase (nirk) from M. tuberculosis bacilli are together involved in the transition to dormancy. As a result, this report could provide a new approach towards the development of anti-tubercular chemotherapy, particularly against the latent form.
MATERIAL AND METHODS
Chemicals and Materials
Foetal Bovine Serum (FBS) and RPMI1640 obtained from Invitrogen Life Technologies, Carlsbad, CA, USA. Dubos broth base and middle brook 7H11-agar base was purchased from BD Difco, USA. All other reagents were purchased from Sigma Aldrich otherwise mention. The stock solution was prepared in distilled water or Dimethyl Sulfoxide.
Cell Lines and Bacterial Cultures
Human acute monocyte leukemia cell line ThP1, purchased from the National Center for Cell Science (NCCS; Pune, India), was maintained in a 25 or 75 cm2 tissue culture flask (Eppendorf, Germany) containing RPMI cell culture medium with 10% heat inactivated FBS. M. tuberculosis H37Ra RFP strain generated as described earlier [22] and grown in Dubos broth containing 50 μg/mL hygromycin under shaking condition at 120rpm at 37o C within a refrigerated shaking incubator (Model 431, Thermo electron Corporation, USA). For the macrophage infection, aerobic or log phase M. tuberculosis H37Ra RFP culture was filtered through an autoclaved 10-11 µm sterile Whatman filter before use [23].
Growth Kinetics of M. Tuberculosis in Macrophage Cells in the Presence of Nitrite
Thp1 cells were infected with M. tuberculosis bacilli by following an earlier method [16]. Thp1 cells (~5 x 104 cells/ well) were seeded in the 96 sterile microplate (Corning, Sigma) containing 200-μL RPMI medium with 20nM of Phorbol Myristic Acetate (PMA). The plates were incubated at 37o C in the presence of 5% CO2 and 95% humidity (Eppendorf, Germany) overnight to differentiate into a macrophage. Macrophage infected with M. tuberculosis H37Ra RFP at 1:1 MOI and incubated further for 10- 12 h. After incubation, uninfected bacilli were removed by three times washing with sterile Phosphate Buffer Saline (PBS) followed by addition of 200 μL RPMI medium. Different concentration of nitrite (5, 10, 20, and 50mM) was added to the 96 microplate wells in triplicate. Plates were further incubated, and the average fluorescence spot count was periodically measured using XTI Array Scan (Thermo fisher,) as already described [23]. DAPI was used on the 8th day of experiment. Macrophage without nitrite acts as a vehicle control.
For the determination of colony count, the infected macrophages were lysed using 200 μL of hypotonic buffer pH 7.4 (10 mM HEPES buffer containing 1.5 mM MgCl2 and 10 mM KCl) in the microplate wells. The 100 μL lysate was then spread on Middle brook 7H11 albumin agar plates to get the CFU after 3 to 4 weeks of incubation at 37°C.
Intracellular Acid-Fast Staining
A modified Ziehl-Neelsen staining was used to detect the acid-fast property of intracellular M. tuberculosis by following an earlier established protocol [24-26]. On the 4th day of infection, medium was aspirated and was fixed with 4% paraformaldehyde (pH 7.4) for 10min, then washed with PBS. Primary stain carbolfuschin added to the staining well, incubated for 5 min. After incubation, washing was done with acid-alcohol for 3mins.
Cells were counterstained with methylene blue for 4 mins. Finally, wells were washed with distilled water and dried the plate. Microscopic images were captured by EVOS microscope using 60X objective.
Inhibition Kinetics of Standard Anti-Tubercular Drugs Against Nitrite Treated Intracellular M. Tuberculosis
Thp1 cells (~5 x 104 cells/well) were seeded and infected as described above. Uninfected bacilli were removed by washing with PBS of pH 7.2, followed by the addition of 200 μL RPMI medium. Immediately after infection (day 0), 10mM of nitrite was added to the microplate and plates were incubated at 37o C in the presence of 5% CO2 and 95% humidity (Eppendorf, Germany). After two days of incubation, standard anti-TB drugs such as Rifampicin (RIF), and Isoniazid (INH) at MIC concentration of 0.052, and 0.053, respectively were added [22]. Average red fluorescence spot intensity was periodically measured by using the XTI Array Scan (Thermo fisher).
Effect of Inos Inhibitor on the Growth of Intracellular M. Tuberculosis
N-(3-(Aminomethyl) benzyl) acetamidine (1400W) is a specific inhibitor of iNOS, binds slowly and tightly to iNOS to develop a powerful inhibition [10]. In order to evaluate the effect of iNOS inhibitor (1400W) on the growth of intracellular M. tuberculosis, solution of two fold serially diluted 1400W inhibitor, at the concentration range (0-10μM), was added to the microplate in triplicate (after washing out the uninfected bacilli) containing infected M. tuberculosis with RPMI medium. The plates were incubated at 37o C in a CO2 incubator with 95% humidity (Eppendorf, Germany). Fluorescence was periodically measured as described earlier [23]. Macrophages infected with M. tuberculosis without iNOS inhibitor acts as a vehicle control.
Effect of Inos Inhibitor on the Growth of Nitrite Induced Dormant Intracellular M. Tuberculosis
Thp1 cells (~5 x 104 cells/well) were seeded in the 96 well sterile microplate in 200 μL RPMI medium with 20nM of PMA. The plates were incubated at 37o C in the presence of 5% CO2 and 95% humidity (Eppendorf, Germany) overnight to differentiate into a macrophage. After incubation, macrophages were infected with M. tuberculosis H37Ra RFP at 1:1 MOI and incubated further for 10-12 h. After PBS wash, RPMI media supplemented with only 10mM of nitrite or 10mM of nitrite along with 5μM concentration of iNOS inhibitor 1400W, in triplicate and plated were incubated at 37o C in the presence of 5% CO2 and 95% humidity (Eppendorf, Germany). Growth was measured as fluorescence spot count as above.
RESULTS
Nitrite Induces Dormancy of M. tuberculosis Within the Macrophage
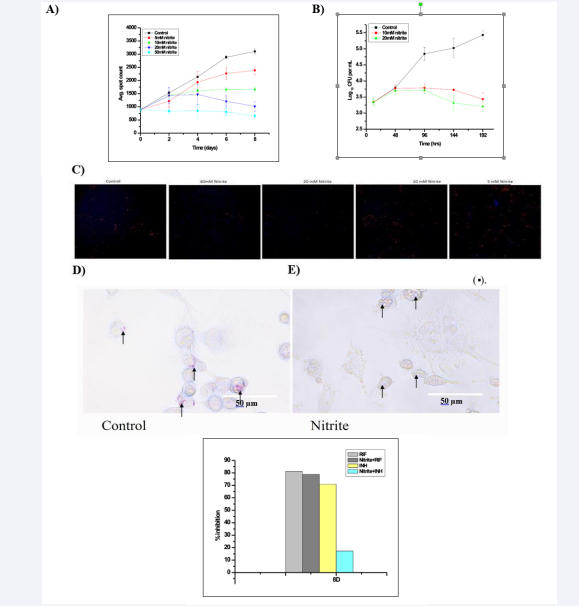
Nitrite treated intracellular M. tuberculosis exhibits growth inhibition and reduced ATP consumption under hypoxic conditions [8]. However, there is no report on the same under normoxic conditions. Thus, to evaluate the effect of nitrite on the intracellular M. tuberculosis, its dose-dependent effect on the bacilli count was observed in infected macrophages (Figure 1A). It was found that there is no significant increase observed in M. tuberculosis spot count (1431.33 ± 305.31 on the 2nd day and the count 1015.01 ± 179.09 on the 8th day actually reduced) upon exposure to nitrite (10 mM). This result is corroborated with microscopy as well as Colony Forming Units (CFU) counts (Figure 1B & C). In fact, cell counts were found to increase up to 48h in both control and 10mM of nitrite treated infected macrophages (from 3.3 ± 0.10 to 3.7 ± 0.10 log10 CFU/mL). Afterwards, counts were significantly decreased with time in the nitrite-treated infected macrophages compared to control up to day 8 (5.43 ± 0.13 to 3.43 ± 0.12 log10 CFU/mL) (Figure 1B). A simultaneous DAPI staining on 8th day clearly indicates that the host cell remains very healthy (Figure 1C). The immediate reduction in the intracellular bacilli counts raised the possibility of developing acquired non-replicative features in them under the influence of nitrite in the medium.
Dormancy Characteristics Developed in Nitrite Treated Intracellular M. tuberculosis
This shifting to a non-replicative dormant state of intracellular bacilli upon nitrite treatment was assessed by Ziehl-Neelsen staining (Figure 1E). The nitrite treated intracellular bacilli showed a pale blue stain, indicative of the loss of the acid-fast property.
Furthermore, it is known that the non-replicative dormant mycobacteria develop drug resistance characteristic [6,9]. We assessed the antibiotic resistance using RIF (an inhibitor which kills bacteria from Active or dormant stage) and INH (an inhibitor which kills only in actively growing stage), applying at their MIC concentrations, and observed that INH (1285.23 ± 263.32) did not show any significant inhibition on the nitrite treated intracellular bacilli (1553.05 ± 150.33) when applied on the 8th day of incubation except RIF (329.14 ± 85.42) (Figure 1F). These results indicated that nitrite-treated intracellular M. tuberculosis bacilli acquired dormancy characteristics.
iNOS Inhibitor Exposure Induces Intracellular Growth of M. tuberculosis Bacilli
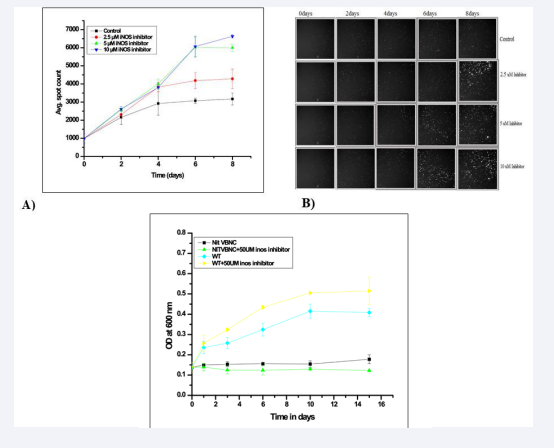
1400W is a specific inhibitor of iNOS without having any significant toxic effect to the mammalian cells [10]. Firstly, the dose response effect of 1400W inhibitor using a concentration range (0-10 μM) on the growth kinetics of M. tuberculosis is observed within infected macrophages (Figure 2A,B). The inhibitor when applied at > 2.5 μM concentration showed significantly increased level of red spot intensities (p = 0.02). The inhibitor effect reaches a plateau at 5 μM concentration. Therefore, we selected 5 μM as optimum concentration of 1400W inhibitor for further experiments. In order to check any potential toxicity on M. tuberculosis cells, 1400W was applied up to 50μM concentration and found to have no inhibitory effect on growth of the bacilli under in vitro conditions (Figure 2C). A parallel exposure of 1400W to extracellular M. tuberculosis cells under identical conditions found that the culture growth remained unaffected even at 50μM concentration of the inhibitor (Figure 2C). So. The effect of 1400W observed on intracellular M. tuberculosis bacilli is specific in nature.
iNOS Inhibition Facilitate the Reactivation of Growth of Nitrite Induced Intracellular Dormant M. tuberculosis
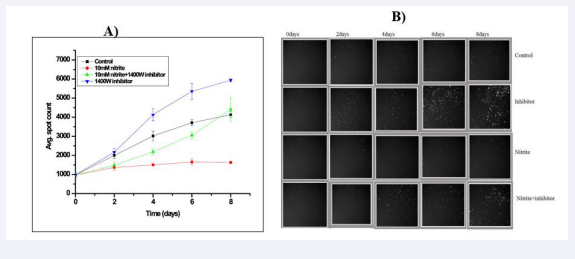
As both macrophages and bacilli could produce NO through their iNOS and nirK enzymes respectively, we were interested to understand the extent of influence exerted either of these two in the bacterial physiology within infected macrophages. It becomes interesting to see the effect of iNOS (1400W) inhibitor on dormant intracellular M. tuberculosis bacilli in presence of nitrite. Therefore, we added 1400W inhibitor after 2 days of nitrite treatment and it was found that nitrite treated intracellular bacilli are showing significantly increased red fluorescence spot count (2317.89 ±228.79 on 6th and 2992.57±349.43 on 8th day) compared to only nitrite treated infected macrophage (1474.27±59.53 on 6th and 1555.74±196.32 on 8th day) respectively with time (Figure 3). This result indicated that the iNOS inhibitor (1400W) facilitate the reactivation of dormant intracellular bacilli even in presence of nitrite in the medium.
DISCUSSION
Many of the earlier studies have clearly inferred that nitric oxide acts as an anti-tubercular agent [11]. Recently, it has been established that nitric oxide acts as an inducer of dormancy in M. tuberculosis cells [6]. It has been reported that M. tuberculosis bacilli could produce NO from nitrite and justifies the reason behind development of nitrite induced dormancy under aerobic condition [6,7]. So, iNOS of macrophage and nir K of M. tuberculosis bacilli together contribute to the total pool of NO produced within the macrophages. Earlier report suggested that nitrite inhibits the intracellular M. tuberculosis growth as well as reduced ATP consumption [8]. Nitric oxide in human is oxidized to produce nitrite and/or nitrate and finally excrete through urine [12]. This conversion depends on other environmental factors [13]. It is well known that NO is converted to nitrite in aqueous medium [14]. Most of the nitric oxide molecules released by activated macrophages are converted to nitrite even in absence of aqueous medium, when the alveolar macrophages are infected with M. tuberculosis bacilli in human lung, which was evidenced from analysis of sputum samples collected from TB patients [15]. A small part of this NO is converted to peroxynitrite after reaction with superoxide produced by M. tuberculosis bacilli which is finally rearranged to nitrate [16]. Anyways, nitrate could act as an alternate respiratory substrate under hypoxic conditions as well as an intracellular substrate for the entry of extracellular nitrite into the bacilli [16,17]. It could be considered that the entry of bacilli in the lung will trigger the release of NO to kill the bacilli in vain. Interestingly, we observed that external nitrite treatment also induced intracellularly growing bacilli to non-replicative dormant stage within the macrophages (Figure 1).
Figure 1: Effect of nitrite on the growth of intracellular Mycobacterium tuberculosis : A) Nitrite Dose dependent growth of intracellular Mtb cells. The average red spot intensity measured at different time (days), B) the average CFU count measured at different time points, C) Intracellular fluorescent spot images: Images were captures on 8th day after addition of DAPI. Each image represents one of the quadrants of the well. Periodically average red spot intensity was measured (HCA, Thermo-fisher) as described in “Material and Methods” section. The results are shown as the mean of three independent experiments ± standard error mean deviation (SEM). Macrophage without nitrite treated as a vehicle control. Effect of nitrite exposure on the acid-fast property of intracellular Mycobacterium tuberculosis H37Ra RFP: Intracellular Mycobacterium tuberculosis were stained with carbolfuschin and counter stained with methylene blue. D) Untreated Intracellular Mycobacterium tuberculosis were used as the control. E) Intracellular Mycobacterium tuberculosis cells treated with nitrite (10 mM). More details are described in the “Materials and Methods” section. Fluorescence microscopic images were captured at 60× objective using an EVOS microscope (Life Technologies). F: Percentage inhibition of standard anti-tubercular drugs against intracellular Mycobacterium tuberculosis: Control and nitrite treated intracellular Mtb H37Ra RFP was treated with drug like RIF (0.05µg/mL), and INZ (0.53µg/mL). Percent inhibition was calculated on 8th day of drug treatment. Rest details are mentioned in “Materials and Methods”. % inhibition = (vehicle control-test/vehicle control-blank) *100 Nitrite treated with drug as a test, and nitrite act as a vehicle control.
Furthermore, acquired characteristic features (loss of acid fastness and antibiotics resistance) also confirmed that nitrite induced dormancy is achieved in the bacilli [18,9]. In our study, we have shown that nitrite treated intracellular bacilli also lose acid fastness characteristic within 2 days of nitrite exposure (Figure 1D,E), otherwise it takes almost 8 days after infection. So, the faster induction of actively growing bacilli to non-replicating stage depends among others, on the concentration of nitrite within the host milieu and bacilli could avail this option as an opportunity to attain early development of dormancy to survive within the host. The resistance to INZ treatment and susceptibility to RIF indicated that intracellular bacilli (Figure 1F) maintained a dormant state throughout the experiment. So, the routes of host NO go to nitrite within the TB infected human lung to make sure that the bacilli develop and maintain latency.
Nevertheless, iNOS/NO production is an important component of the host defence strategy against any infection. Herein, we observed that inhibition of host iNOS enhances the growth of intracellular M. tuberculosis bacilli (Figure 2).
Figure 2: Effect of 1400 W on the growth of intracellular Mycobacterium tuberculosis: Different concentration of iNOS inhibitor (1400W) (2.5, 5, 10 µM) was added in each well after removal of extracellular bacilli and further incubated at 37 o C, 5% CO2 atmosphere. Periodically average red spot intensities were measured (HCA, Thermo-fisher) as explained in “Material and Methods” section. The data represent as means of the triplicate results with ± SD. B) Intracellular fluorescent spot images: Images were captures during the read and processed using Cellomics software. Each image represents one of the quadrants of the well. C: Effect of 1400 W on the growth of extracellular Mycobacterium tuberculosis:1400W was added at 50 µM concentration in each triplicate well of VBNC and wild type Mtb culture and further incubated at 37 o C. Periodically optical density were measured as explained in “Material and Methods” section. The data represent as means of the triplicate results with ± SD.
Earlier report also suggests that iNOS induced in murine macrophage due to the infection of M. tuberculosis and protected the host whereas avoidance of NO is needed for M. tuberculosis survival [19-21]. Martin, et al also reported that the NO exposure to M. tuberculosis under in vitro culture conditions induces dormancy via up regulation of the dormancy regulon genes [17]. Interestingly, our robust experimental conditions allow controlled manipulation of the iNOS/nirK function to understand their precise influence on the intracellular bacilli. The application of iNOS inhibitor is also found to break the nitrite induced dormancy of intracellular bacilli (Figure 3).
Figure 3: Effect of iNOS inhibitor on growth of nitrite induced intracellular dormant Mycobacterium tuberculosis bacilli: Macrophages were infected with actively growing Mtb H37Ra RFP cultures with ~1:1 MOI. After 10h of incubation extracellular bacilli were washed with 1X PBS for 3 times, followed by addition of 10mM of nitrite, 10mM of nitrite along with 5 μM 1400 W, 5 μM 1400W only was added in each triplicate well. The plate was further incubated at 37 o C, 5% CO2 atmosphere. A) Periodically red spots intensities were measured (HCA, Thermo-fisher) as explained in “Material and Methods” section. The data represent as means of the triplicate results with ± SD. B) Intracellular fluorescent spot images: Images were captures during the read and processed using Cellomics software. Each image represents one of the quadrants of the well.
This clearly indicated that intervention of host NO stress could lead to the reactivation of the dormant intracellular bacilli even in presence of nitrite. As, host ions’ is the source of total nitrite pool in the lung, iNOS inhibitor lead to the decrease in nitrite dependent NO production in lungs to destabilize the maintenance of non-replicating status of the bacilli. Graphical representation of (Figure 4) shows the reactivation of M. tuberculosis with inhibition of host NO production.
Figure 4: Graphical representation of reactivation of dormant Mycobacterium tuberculosis: In macrophage, iNOS induces NO production from arginine as a substrate. NO of both the mycobacteria and host macrophage system helps to maintain dormancy characteristics of M. tuberculosis within the infected macrophage. Upon inhibition of host iNOS using 1400W inhibitor dormant mycobacteria starts replicating.
In summary, nitrite induced dormancy of intracellular M. tuberculosis cells is confirmed by CFU, loss of acid fastness, and drug resistance towards INH. Also, inhibition of macrophage NO production regained the active state of M. tuberculosis cells inside the macrophages. This finding could be useful to redesign and shorten the therapeutic treatment against TB.
FUNDING
This work was supported by the Council of Scientific and Industrial Research (CSIR), India, through a grant in aid projects BSC0103 and CSC0406 to DS.
ACKNOWLEDGMENT
SA and AY are thankful to UGC and DST-INSPIRE for their research fellowships, respectively.
Author Contributions
The study was carried out by SA. SA wrote the original draft and AY helped to analyze the data. Overall supervision is provided by DS.
REFERENCE
- Houben RM, Dodd PJ. The global burden of latent tuberculosis infection: a re-estimation using mathematical modelling. PLoS Med. 2016; 13: e1002152.
- https://www.who.int/europe/multi-media/item/together--we-can-end-tb-by-2035
- Heidary M, Shirani M, Moradi M, Goudarzi M, Pouriran R, Rezaeian T, et al. Tuberculosis challenges: Resistance, co-infection, diagnosis, and treatment. Eur J Microbiol Immunol (Bp). 2022; 12: 1-17.
 4.Choi HS, Rai PR, Chu HW, Cool C, Chan ED. Analysis of nitric oxide synthase and nitrotyrosine expression in human pulmonary tuberculosis. Am J Respir Crit Care Med. 2002; 166: 178-218.
4.Choi HS, Rai PR, Chu HW, Cool C, Chan ED. Analysis of nitric oxide synthase and nitrotyrosine expression in human pulmonary tuberculosis. Am J Respir Crit Care Med. 2002; 166: 178-218.- Vitek MP, Brown C, Xu Q, Dawson H, Mitsuda N, Colton CA. Characterization of NO and cytokine production in immune-activated microglia and peritoneal macrophages derived from a mouse model expressing the human NOS2 gene on a mouse NOS2 knockout background. Antioxid Redox Signal. 2006; 8: 893-901.
- 6.Gample PS , Agrawal S, SarkarD .Evidence of nitrite acting as a stable and robust inducer of non-cultivability in Mycobacterium tuberculosis with physiological relevance. Sci Rep. 2019; 9: 9261.
- Agrawal S, Gample S, Yeware A, Dhiman Sarkar. Novel gene similar to nitrite reductase (NO forming) plays potentially important role in the latency of tuberculosis. Sci Rep. 2021; 11: 19813.
- 8.Cunningham-Bussel A, Zhang T, Nathan CF. Nitrite produced by Mycobacterium tuberculosis in human macrophages in physiologic oxygen impacts bacterial ATP consumption and gene expression. Proc Natl Acad Sci U S A. 2013; 110: E4256-E4265.
- Sikri K, Duggal P, Kumar C, Batra SD, Vashist A, Bhaskar A, et al. Multifaceted remodeling by vitamin C boosts sensitivity of Mycobacterium tuberculosis subpopulations to combination treatment by antitubercular drugs. Redox Biol. 2018; 15:452-466.
- Garvey EP, Oplinger JA, Furfine ES, Kiff RJ, Laszlo F, Whittle BJ, et al. 1400W is a slow, tight binding, and highly selective inhibitor of inducible nitric-oxide synthase in vitro and in vivo. J Biol Chem. 1997; 272: 4959-4963.
- Jamaati H, Mortaz E, Pajouhi Z, Folkerts G, Movassaghi M, Moloudizargari M, et al. Nitric Oxide in the Pathogenesis and Treatment of Tuberculosis. Front Microbiol. 2017; 8: 2008.
- Lundberg J, Weitzberg E, Gladwin M. The nitrate–nitrite–nitric oxide pathway in physiology and therapeutics. Nat Rev Drug Discov. 2008; 7: 156-167.
- MacMicking J, Xie Q-W, Nathan C. Nitric oxide and macrophage function. Annu. Rev. Immunol. 1997; 15: 323-350.
- 14. Kelm M. Nitric oxide metabolism and breakdown. Biochim Biophys Acta. 1999; 1411: 273-289.
- Sarkar D. Nitrite reductase (NIRB) as potential antitubercular target and a method to detect the severity of tuberculosis disease. US9670523B2. 2017.
 Sarkar S, Sarkar D. Potential use of nitrate reductase as a biomarker for the identification of active and dormant inhibitors of Mycobacterium tuber-culosis in a THP1 infection model. J Biomol Screen 2012; 17: 966-973.
Sarkar S, Sarkar D. Potential use of nitrate reductase as a biomarker for the identification of active and dormant inhibitors of Mycobacterium tuber-culosis in a THP1 infection model. J Biomol Screen 2012; 17: 966-973.- 17.Martin I Voskuil, Dirk Schnappinger, Kevin C Visconti, Maria I Harrell, Gregory M Dolganov, David R Sherman, et al. Inhibition of Respiration by Nitric Oxide Induces a Mycobacterium tuberculosis Dormancy Program. J Exp Med. 2003; 198: 705-713.
- Deb C, Lee C-M, Dubey VS, Daniel J, Abomoelak B, Sirakova TD, et al. A Novel In Vitro Multiple-Stress Dormancy Model for Mycobacterium tuberculosis Generates a Lipid-Loaded, Drug-Tolerant, Dormant Pathogen. PLoS One. 2009; 4: e6077.
- Chan J, Tanaka K, Carroll D, Flynn J, Bloom BR. Effects of nitric oxide synthase inhibitors on murine infection with Mycobacterium tuberculosis. Infect Immun. 1995; 63:736-740.
- 20.Scanga CA, Mohan VP, Tanaka K, Alland D, Flynn JL, Chan J. The inducible nitric oxide synthase locus confers protection against aerogenic challenge of both clinical and laboratory strains of Mycobacterium tuberculosis in mice. Infect Immun. 2001; 69:7711- 7717.
- 21.Kaufmann SH, Cole ST, Mizrahi V, Rubin E & Nathan C. Mycobacterium tuberculosis and the host response. J Exp Med. 2005; 201: 1693-1697
- Yeware A, Sarkar D. Novel red fluorescence protein based microplate assay for drug screening against dormant Mycobacterium tuberculosis by using paraffin. Tuberculosis (Edinb). 2018; 110: 15-19.
- Yeware A, Agrawal S, Sarkar D. A high content screening assay for identifying inhibitors against active and dormant state intracellular Mycobacterium tuberculosis. J Microbiol Methods. 2019; 164: 105687.
- Koch ML, COTE RA. COMPARISON OF FLUORESCENCE MICROSCOPY WITH ZIEHL-NEELSEN STAIN FOR DEMONSTRATION OF ACID-FAST BACILLI IN SMEAR PREPARATIONS AND TISSUE SECTIONS. Am Rev Respir Dis. 1965; 91: 283-284.
- Lahiri KK, Chatterjee SK. a simple cold staining method for acid fastbacilli. Med J Armed Forces India. 1994; 50: 256-258.
- Chen P, Shi M, Feng GD, Liu JY, Wang BJ, Shi XD, et al. A highly efficient Ziehl-Neelsen stain: identifying de novo intracellular Mycobacterium tuberculosis and improving detection of extracellular M. tuberculosis in cerebrospinal fluid. J Clin Microbiol. 2012; 50: 1166-1170.