Subtenon Implantation of Wharton’s Jelly Derived Mesenchymal Stromal Cell for Retinitis Pigmentosa: A 1–2 Year Follow-Up Report
- 1. 23 Century International Life Science Centre, Malaysia
- 2. Department of Ophthalmology Hospital Shah Alam, Malaysia
Abstract
Background: Retinitis Pigmentosa (RP) is a group of genetically inherited retinal dystrophies characterized by the progressive degeneration of photoreceptors, leading to irreversible vision loss. With limited therapeutic options currently available, stem cell therapy has emerged as a promising regenerative strategy for retinal repair and vision restoration.
Objective: The study aimed to evaluate the safety, feasibility, and potential therapeutic effects of subtenon implantation of human Wharton’s Jelly-derived Mesenchymal Stem
Cells (WJ-MSCs) by inhibiting proinflammatory cytokine expression, thereby suppressing chronic retinal inflammation and preventing apoptosis of photoreceptor cells.
Materials and Methods: We present four case illustrations of patients diagnosed with RP who were treated with WJ-MSCs injected in the deep subtenon space. Each patient received a total of four injection sessions between November 2020 and January 2023. The procedure was well tolerated in all sessions, and patients were monitored closely for clinical outcomes and potential adverse effects.
Result: Patients 1 and 2 underwent a more intensive treatment regimen with 7 subtenon WJ-MSC injection over a 24-month period. This approach aimed to provide sustained therapeutic effects, with both patients reporting stable visual improvements and no adverse events during the follow-up period. Moreover, both patients 3 and 4 received a shorter treatment course with 3 subtenon WJ- MSC injection over 12 months. Despite the reduced number of injection, both patients experienced intermittent episodes of brighter vision, suggesting potential therapeutic benefits. The study found significant improvements in best corrected visual acuity, outer retinal thickness, and full-field electroretinography parameters, supporting the potential benefits of this treatment modality. Our study involving 4 patients with RP demonstrated that subtenon administration with hWJ-MSC treatment was found to be effective and safe, with no serious adverse events or ophthalmic/systemic side effects reported during the follow- up period.
Conclusion: These findings align with previous study, supporting the safety and efficacy of subtenon hWJ-MSC administration in RP patients and suggesting it as a promising therapeutic approach.
Keywords
• Retinitis Pigmentosa
• Wharton’s Jelly-derived Mesenchymal Stem Cells
• Subtenon implantation
• Visual impairment
Citation
Khairullah NS, Ngah NF, Aziz RAA, Teh D, Tiah A (2025) Subtenon Implantation of Wharton’s Jelly Derived Mesenchymal Stromal Cell for Retinitis Pigmentosa: A 1 – 2 Year Follow-Up Report. JSM Ophthalmol 12(1): 1097.
INTRODUCTION
Retinitis pigmentosa (RP) is a significant cause of visual impairment in Malaysia, particularly among younger individuals. This hereditary retinal disorder leads to progressive vision loss due to the degeneration of photoreceptor cells in the retina [1,2].
A study at the University of Malaya Medical Centre found that RP accounted for 10.04% of cases of low vision and blindness, ranking it among the top four causes in their urban patient population. Similarly, research from Miri Hospital in Sarawak reported RP as the third leading cause of irreversible visual impairment, affecting 9.7% of patients. Nationally, RP is a major contributor to childhood blindness. A study of blind school students indicated that37.7% of inherited childhood blindness cases were due to RP, often linked to consanguinity marriages [3,4]. The inheritance patterns of RP include autosomal dominant, autosomal recessive, and X-linked forms. Malaysia’s diverse ethnic composition, including Malay, Chinese, Indian, and indigenous groups, contributes to a rich genetic landscape. This diversity may influence the prevalence and types of genetic mutations associated with RP in different populations. For instance, certain mutations may be more common in specific ethnic groups, affecting the clinical presentation and progression of the disease. Understanding the specific genetic mutations prevalent in Malaysian populations is crucial for accurate diagnosis, genetic counselling, and the development of targeted therapies [3,4].
In response to the challenges posed by RP, Malaysia has seen advancements in medical research and treatment options. Stem cell therapy has emerged as a potential treatment for RP, offering hope for patients with limited options. We, 23 Century International Life Science Centre are exploring the use of stem cells to repair damaged retinal tissue, aiming to halt or even reverse the progression of the disease.
This paper presents a continuation of the initial clinical experience jointly reported by 23 Century Medical Group and Hospital Shah Alam, which demonstrated early functional improvements and safety following subtenon administration of UC-MSCs in RP patients. Building upon these preliminary outcomes, the current study aims to further evaluate the long-term efficacy, safety, and vision related quality of life improvements in a larger patient cohort. By expanding the clinical dataset and refining the treatment protocol, this study seeks to establish subtenon UC-MSC therapy as a viable adjunct in the management of RP, offering new hope for a condition long considered untreatable. The trial, conducted at Hospital Shah Alam between November 2020 and January 2023.
The Ministry of Health (MOH) Malaysia has recognized the potential of mesenchymal stem cells in treating RP and other degenerative retinal diseases. In 2022, the ministry issued guidelines to ensure the safe and ethical use of stem cell therapies in clinical settings [5]. While stem cell therapy for RP is still in the research and clinical trial stages, the progress made in Malaysia offers hope for patients with limited treatment options. Continued research and adherence to regulatory standards will be crucial in determining the long-term efficacy and safety of these therapies.
In summary, Malaysia is at the forefront of exploring stem cell therapy as a treatment for RP. With ongoing clinical trials and regulatory support, there is optimism that this approach may provide new avenues for managing and potentially reversing the effects of this debilitating condition.
MATERIALS & METHODS
This clinical study involved eight eyes from four patients who attended the Ophthalmology Clinic at Shah Alam Hospital between November 2020 and January 2023. The study was conducted in accordance with the tenets of the Declaration of Helsinki. All patients were fully informed regarding the study’s purpose, procedures, and course. Written informed consent was obtained from each subject prior to the initiation of any study related assessments or procedures. The diagnosis of retinitis pigmentosa (RP) was established based on clinical history, ophthalmological examination findings, visual field (VF) testing, and optical coherence tomography (OCT) results.
The inclusion criteria were as follows: (1) a clinical diagnosis of retinitis pigmentosa (RP) confirmed by clinical history, fundus appearance, visual field (VF) testing, optical coherence tomography (OCT), and electroretinography (ERG); (2) age greater than 18 years; (3) ability to perform a reliable VF evaluation; and (4) availability of at least one year of follow-up data.
Patients were excluded if they had a history of ocular surgery other than cataract extraction; ocular media opacities that compromised image quality or interfered with test results; coexisting ocular conditions such as retinal pathology unrelated to RP, glaucoma, uveitis, strabismus, or nystagmus; or systemic diseases, including diabetes, neurological disorders, or hypertension, that could affect the study outcomes.
All surgical procedures and ophthalmic assessments were performed by a single experienced vitreoretinal surgeon (AO). Baseline ophthalmic evaluations included best-corrected visual acuity (BCVA), applanation tonometry, slit-lamp biomicroscopy, color fundus photography, OCT, and VF assessment.
OCT was conducted using the Optovue system (Optovue Inc., USA) with a standardized scanning protocol. Visual field testing was performed using the Humphrey Field Analyzer (Threshold 30-2, HFA II 750; Carl Zeiss Meditec AG, Germany). Electroretinography (ERG) recordings were obtained using the ERG-Vision monitor (Monpack 3, Metrovision, France), following the standards set by the International Society for Clinical Electrophysiology of Vision (ISCEV). All tests were conducted with the same instruments and by the same trained technician to ensure consistency.
Preparation Of Umbilical Cord-Derived Mesenchymal Cell (MSC)
The WJ-MSCs used in this study were isolated from Wharton’s Jelly of the umbilical cord, collected from a single donor with informed maternal consent. The umbilical cord, initially immersed in a cord preservative solution, was washed with 0.9% sodium chloride injection and soaked in 70% alcohol for disinfection. After a final rinse with 0.9% sodium chloride, the cord was measured by clamping and stretching both ends with hemostats, then cut into 2 to 3 cm segments.
The tissue was subsequently weighed, cut, washed, and centrifuged prior to the culturing process in complete culture medium. The cultured tissues were incubated in a CO2 incubator maintained at 37°C, 5.0% CO2 , and 95% relative humidity. All cell preparation and cultivation procedures were conducted by Beike 23 Century International Stem Cell Laboratory (Beike 23C), a facility accredited by the Ministry of Health (MOH) for cGMP and cGTP compliance.
Culture-expanded cells were cryopreserved at passage 4 (P4) using standard cryopreservation protocols until use. The cryopreserved cells were characterized via flow cytometry in accordance with the International Society for Cell & Gene Therapy (ISCT) criteria for mesenchymal stem cells. Positive expression of surface markers CD90, CD73, and CD105 was required to be ≥95%. CD29 was included as an additional marker to verify the identity of the MSC culture, as part of Beike 23C’s characterization protocol. Concurrently, negative expression (≤2%) of CD45, CD34, CD79a, CD14, and HLA-DR was confirmed.
Quality control analyses including sterility testing, mycoplasma detection, and bacterial endotoxin testing were conducted prior to product release. The average cell viability exceeded 90%, and each patient received 10 to 11 million viable cells suspended in 1.5 mL of electrolyte solution per eye.
Injection of WJ-MSC
A total of 1.5mL of Wharton’s Jelly-derived mesenchymal stromal cell (WJ-MSC) suspension was administered into the subtenon space of each eye. The procedure was performed under local anaesthesia using proparacaine hydrochloride eye drops (Alcaine®, Alcon, USA) under sterile conditions. A 25G curved subtenon cannula (BD Visitec™, UK) was used to deliver the suspension into either the superotemporal or inferonasal region to ensure effective distribution. Post-operatively, patients were prescribed Guttae Maxitrol® (neomycin and dexamethasone) eye drops to be applied four times daily for one week. Oral Ibuprofen 200 mg was administered three times daily for one week, and amoxicillin-clavulanate 500 mg was given four times daily for three days as prophylactic therapy.
RESULTS
Clinical Outcomes of Subtenon MSC Administration in Four Patients 1 and 2.
Both patients received a total of seven mesenchymal stem cell (MSC) transplantations, administered every 4-6 weeks, with follow-up from November 2020 to November 2022. Both patients reported no deterioration in vision since the first subtenon implantation in November 2020. Optical coherence tomography (OCT) images consistently demonstrated the maintenance of a hyperreflective region, with no notable increase in intensity. The previously reported improvements in visual function remained stable throughout the two-year follow-up, with no evidence of regression (Figures 1,2).
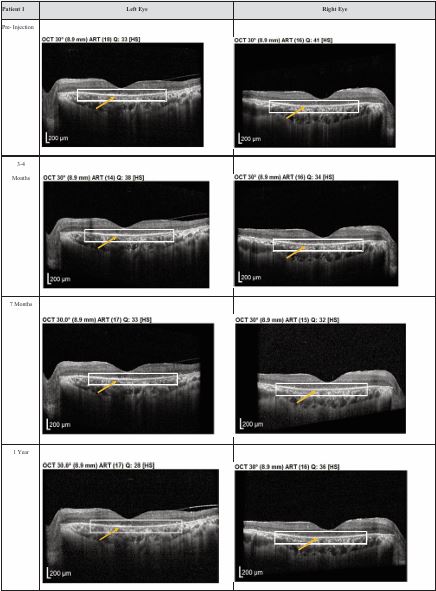
Figure 1 Optical coherence tomography (OCT) images for both eyes of Patient 1 from pre-injection up to 1 year follow-up. Orange arrow demonstrates the presence of hyperreflective material at the interdigitation area of the photoreceptors at the macula and extramacular region. These were observed and maintained until the last follow-up examination.
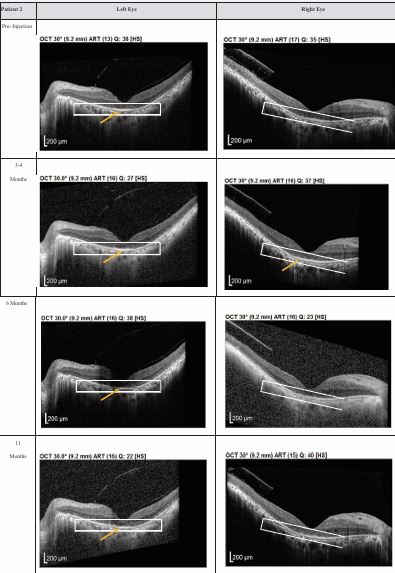
Figure 2 Optical coherence tomography (OCT) images for both eyes of Patient 2 from pre-injection up to 11 month follow- up. Orange arrow demonstrates the presence of hyperreflective material at the interdigitation area of the photoreceptors at the macula and extramacular region. These were observed and maintained until the last follow-up examination.
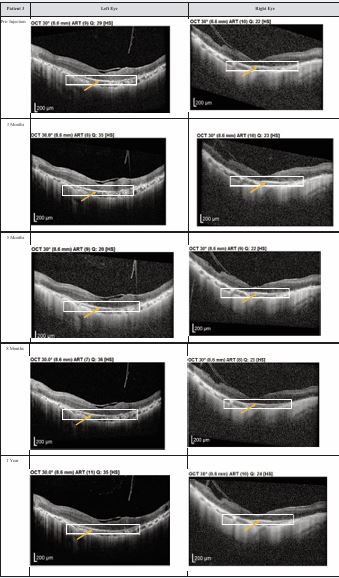
Figure 3 Optical coherence tomography (OCT) images for both eyes of Patient 3 from pre-injection up to 1 year follow-up. Orange arrow demonstrates the presence of hyperreflective material at the interdigitation area of the photoreceptors at the macula and extramacular region. These were observed and maintained until the last follow-up examination.
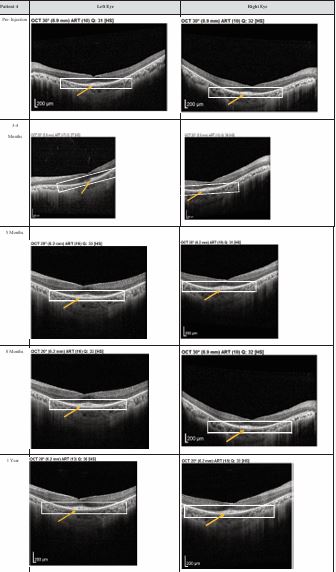
Figure 4 Optical coherence tomography (OCT) images for both eyes of Patient 4 from pre-injection up to 1 year follow-up. Orange arrow demonstrates the presence of hyperreflective material at the interdigitation area of the photoreceptors at the macula and extramacular region. These were observed and maintained until the last follow-up examination.
Table 1: Summary of clinical outcomes of subtenon MSC administration in 4 patients.
|
Clinical Outcome |
Patients 1 & 2 |
Patients 3 & 4 |
|
Number of MSC Injections |
7 injections (every 4-6 weeks) |
3 injections (every 4-6 weeks) |
|
Follow-up Period |
November 2020 - November 2022 (2 years) |
December 2022 - November 2023 (1 year) |
|
Vision Outcome |
No deterioration in vision reported |
Intermittent brighter vision reported, persistent through follow-up |
|
OCT Findings |
Maintenance of hyperreflective region, no intensity increase |
Pronounced layer of photoreceptors, hyperreflective material at PRC interdigitation zone at both macula and extramacular regions |
|
Visual Function |
Improvements in visual function remained stable, no regression |
Improvement in brightness, corresponding with OCT findings |
|
Safety Profile |
No severe ophthalmic or systemic complications |
No severe ophthalmic or systemic complications |
|
Adverse Events |
None reported |
None reported |
|
OCT Structural Changes |
Stable, no progression |
Positive structural changes in photoreceptor layer |
|
Follow-up Frequency |
Every 4-6 weeks for MSC injections; then biannual follow-ups |
Every 3-4 months post 3 MSC injections |
|
Overall Conclusion |
Stable visual function and safety over two years |
Subjective improvement in vision, structural changes observed |
DISCUSSION
Mesenchymal stem cells (MSCs) are multipotent stromal cells capable of self-renewal and differentiation into various mesenchymal tissues, including bone, cartilage, and adipose. Additionally, MSCs can differentiate into retinal progenitor cells, photoreceptors, and retina neural-like cells. They also exert neuroprotective and pro- regenerative effects by releasing paracrine factors that support tissue repair and protect against retinal damage [6].
In animal models, Wharton’s Jelly-derived Mesenchymal Stem Cells (WJ-MSCs) have shown the ability to stimulate retinal progenitor cells and activate self-repair mechanisms in the retina. These improvements are likely driven by the paracrine effects of the implanted cells, which promote functional integration with the host retina. This integration helps substitute lost retinal photoreceptors or maintain the neuroprotective and neurotrophic effects necessary to preserve the function of existing photoreceptors in the recipient [7,8]. Recent advances in regenerative medicine have introduced mesenchymal stem cells (MSCs) as a promising therapeutic strategy for neurodegenerative retinal conditions. MSCs, particularly those derived from human umbilical cord (UC-MSCs), offer distinct advantages including low immunogenicity, ethical acceptability, and a potent paracrine profile conducive to neuroprotection, anti inflammation, and angiogenesis. The subtenon route, being minimally invasive, has gained traction for its safety and proximity to the posterior segment, enabling sustained trophic support to degenerating retinal cells. Subtenon MSC administration appears to be safe and well-tolerated, with no serious complications across all four patients. The treatment led to stable or improved visual outcomes, with objective optical coherence tomography (OCT) imaging changes aligning with subjective improvements, particularly in patients with shorter follow-up.
CONCLUSION
Subtenon administration of human Wharton’s Jelly derived mesenchymal stem cells (WJ-MSCs) in patients with retinitis pigmentosa (RP) is hypothesized to facilitate retinal repair through immunomodulatory mechanisms, primarily by downregulating proinflammatory cytokines within the retinal microenvironment. This may attenuate chronic inflammation and inhibit apoptosis of retinal cells [6].
Clinical observations to date indicate sustained visual function without evidence of disease progression or adverse effects, supporting both the safety and potential efficacy of subtenon WJ-MSC transplantation. Although stem cell-based regenerative therapy demonstrates promise, extended follow-up is essential to evaluate the long-term stability of functional improvements and to establish optimal intervals for repeat dosing. Considering the progressive nature of RP and the outcomes observed in this case series, an initial treatment course comprising 3-4 subtenon injections, followed by maintenance booster doses at 6-12 months interval, may represent an effective strategy for prolonging therapeutic benefit.
ACKNOWLEDGEMENT
We would like to thank the Director General of Health Malaysia for allowing the publication of this manuscript and the staff of Department of Ophthalmology of Shah Alam Hospital for their contribution and support to this study. We also thank the Chief Executive Officer of 23 Century International Life Science Centre for her continuous support in this case study.
REFERENCES
- Khairullah SN, Fariza NN, Roslin AAZ, Angelina T. Subtenon implantation of Wharton’s jelly-derived mesenchymal stromal cells in retinitis pigmentosa. Med J Malaysia. 2022; 77: 564-568.
- Reddy SC, Tan BC. Causes of childhood blindness in Malaysia: results from a national study of blind school students. Int Ophthalmol. 2001; 24: 53-59.
- Reddy SC, Tajunisah I, Low KP, Karmila AB. Prevalence of eye diseases and visual impairment in urban population–a study from University of Malaya Medical Centre. Malays Fam Physician. 2008; 3: 25.
- Kevin-Tang XH, Tajunisah I, Lott PPW, Reddy SC. Prevalence of visual impairment and eye diseases in Malaysia: A cross-sectionalprospective study at the University of Malaya Medical Centre. Malays Fam Physician. 2024; 19: 30.
- Roza S, Foo SS, Izzuna MMG. Mesenchymal stem cells for retinitis pigmentosa and other degenerative retina disease. 2022.
- Zarbin M. Cell-based therapy for degenerative retinal disease. Trends Mol Med. 2016; 22: 115-134.
- Liang X, Ding Y, Zhang Y, Tse HF, Lian Q. Paracrine mechanisms of mesenchymal stem cell-based therapy: current status and perspectives. Cell Transplant. 2014; 23: 1045-1059.
- Kyurkchiev D, Bochev I, Ivanova-Todorova E, Mourdjeva M, Oreshkova T, Belemezova K, et al. Secretion of immunoregulatory cytokines by mesenchymal stem cells. World J Stem cells. 2014; 6: 552.