Metabolic Effects of Dissolved Aspartame in the Mouth before Meals in Prediabetic Patients; a Randomized Controlled Cross-Over Study
- 1. Department of Endocrinology and Metabolism, Manisa State Hospital, Turkey
- 2. Department of Endocrinology and Metabolism, School of Medicine, Akdeniz University, Turkey
Abstract
In Type 2 Diabetes Mellitus, incretin axes play an important role in terms of progressive beta abnormalities. It was found that enteroendocrin cells in the small bowel have T1R2 and T1R3 taste receptors. Artificial sweeteners and glucose have significant effects on secretion of GLP-1 and GIP hormones from intestine. Recent research studies worked on healthy people showed that glucose triggers release of these hormones by the taste receptors in the L cells. The aim of this study was evaluation of the metabolic effects of dissolved aspartame in the mouth taken before meals in prediabetic patients.
This cross-over study was done in Akdeniz University, Endocrinology and Metabolism Unit. 54 prediabetic patients were included to this study. Patients were randomly separated to two groups. At the beginning of the study, patients interviewed with specialized diabetes dietitians. The first group was initiated with diet for three months and continued with diet and aspartame during second three months. Diet and aspartame were started in the second group for the first three months. Aspartame was discontinued after three months and patients continued with only diet during the second three months. Two tablets of aspartame (1 tablet equal to 18 mg) before meals were taken by dissolving on the tongue.In the two groups weight, height, BMI, waist circumference, fasting and postprandial blood glucose, hemoglobin A1c (HbA1c), fructosamine, alanine aminotransferase (ALT), gamma-glutamyltransferase (GGT), blood urea nitrogen (BUN), creatinine, LDL cholesterol, HDL cholesterol, triglycerides and insulin were evaluated at 0, 3 and 6 months.
In the first group, diet was found effective on losing weight at the end of the third month. After aspartame was added, weight loss continued till the end of the 6 month. In the second group, weight loss was detected with aspartame and diet during the first three months. However; in the second three months, weight gain occurred after aspartame was discontinued. Both groups were compared regarding weight loss in the first and second period. Weight loss in the first group was greater than in the second group during second period (p=0.027). The changes in the other parameters were not found significantly different.
In conclusion, aspartame has additional effect on weight loss in prediabetic patients. We need long term studies to investigate the relation between weight change and incretins.
Keywords
• Aspartame
• Weight
• Incretin
• Prediabetic patients
Citation
Koyuncu BU, Balc? MK (2014) Metabolic Effects of Dissolved Aspartame in the Mouth before Meals in Prediabetic Patients; a Randomized Controlled Cross-Over Study. J Endocrinol Diabetes Obes 2(2): 1032.
INTRODUCTION
Diabetes Mellitus (DM) is a group of metabolic diseases characterized by hyperglycemia due to defective insulin secretion, insulin effect, or both [1]. There is a group of patients with glucose levels higher than normal, but not high enough to be considered diabetes [2]; these patients have Impaired Fasting Glucose (IFG) and impaired glucose tolerance (IGT), both of which are referred to as prediabetes [3]. IFG and IGT are stages of the natural course of impaired glucose metabolism, and can develop into any type of diabetes [4]. In type 2 DM incretin axis abnormalities were reported to play an important role in progressive beta cell insufficiency [5].
The sense of taste was once thought to exist only in the taste cells of the mouth; however, recent research has shown that enteroendocrine cells in the bowels secrete hormones, and include T1R2 and T1R3 receptors that sense taste. Apart from glucose, artificial sweeteners are sensed by the bowels as glucose and lead to the secretion of GLP-1 (glucagon-like peptide-1) and GIP (glucose-dependent insulinotropic polypeptide) hormones; therefore, an increase in these hormones increases glucose absorption in the bowels. Such knowledge inspired research on modulating GLP-1 secretion in intestinal taste cells in the search for new drugs for treating obesity and diabetes. Nonetheless, the possibility of other mechanisms in the intestine and other organs that play a role in sensing glucose has been reported [6,7].
Recent experimental trials performed in healthy individuals using artificial sweeteners along with glucose showed an increase in GLP-1 secretion, and suggest that it might occur via the taste receptors in L cells [6,8]. The aim of this study was evaluation of the metabolic effects of dissolved aspartame in the mouth taken before meals in prediabetic patients.
MATERIALS AND METHODS
This cross-over study was done in Akdeniz University, Endocrinology and Metabolism Unit. 54prediabetic patients were included to this study. The study included 54prediabetic patients that were diagnosed according to American Diabetes Association (ADA) criteria. Patients with a plasma glucose level of 100-125 mg dL–1 following 8-12 h of fasting and/or an oral glucose tolerance test (OGTT) 2nd-h blood glucose value of 140- 199 mg dL–1 were included. Exclusionary criteria were age <20 years, use of any medication that could interfere with blood sugar regulation and/or an active infection or disease such as hyperthyroidism, and females planning to get pregnant.
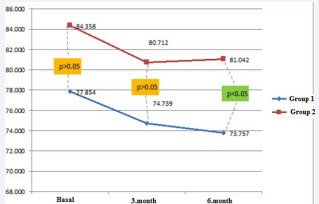
Patients were randomly separated to two groups. At the beginning of the study, patients interviewed with specialized diabetes dietitians. The first group was initiated with diet for three months and continued with diet and aspartame during second three months. Diet and aspartame were started in the second group for the first three months. Aspartame was discontinued after three months and patients continued with only diet during the second three months (Figure1).
Figure 1: Mean weight over time in the 2 study groups
Two tablets of aspartame (1 tablet equal to 18 mg) before meals were taken by dissolving on the tongue.
In the two groups weight, height, BMI, waist circumference, fasting and postprandial blood glucose, Hemoglobin A1c (HbA1c), fructosamine, alanine aminotransferase (ALT), Gamma-Glutamyl Transferase (GGT), Blood Urea Nitrogen (BUN), creatinine, LDL cholesterol, HDL cholesterol, triglycerides and insulin were evaluated at 0, 3 and 6 months.
Statistical analysis
Statistical analysis was performed using SPSS v.18.0 for Windows. The normality of distribution of numeric variables was analyzed via the Kolmogorov-Simirnovtest. For data with normal distribution definitive statistics are given as mean ± SE and median (range), whereas categorical data are expressed as number and percentage. In groups with normal distribution the t test was used for parametric tests and the Mann-Whitney U test was used in the absence of normal distribution. The results were analyzed at the 95% confidence interval (CI) and P < 0.05 was accepted as statistically significant.
RESULTS
In the first group, diet was found effective on losing weight at the end of the third month. After aspartame was added, weight loss continued till the end of the 6 month. In the second group, weight loss was detected with aspartame and diet during the first three months. However; in the second three months, weight gain occurred after aspartame was discontinued. Both groups were compared regarding weight loss in the first and second period. Weight loss in the first group was greater than in the second group during second period (p = 0.027). Body mass changes of each group in the third and sixth months were observed. Body mass changes in the third month (? mass 3); were obtained by sub stracting basal weights from 3rd months weights. Body mass changes in the sixth month (? mass 6) were obtained by sub stracting third months weights from sixth months weights, and by substracting basal weight from sixth months weights total body mass changes were obtained (? mass total). No statistical differences were observed in the comparison of body mass changes of each group (? mass) (Table 1).
Table 1: Demographic data and weight change in the 2 study groups.
| Group 1 | Group 2 | P | |
| N | 28 | 26 | |
| Gender (F/M) | 16/12 | 24/2 | |
| Age (years) | 53.4 ± 9.4 | 50.7 ± 10.2 | |
| Basal Weight (kg) | 77.9 ± 13.1 | 84.4 ± 12.0 | NS |
| Change in Weight After 3 Months (kg) | 74.7 ± 12.0 | 80.7 ± 11.2 | NS |
| Change in Weight After 6 Months (kg) | 73.8 ± 11.5 | 81.0 ± 11.9 | 0.027 |
NS: Not significant (P > 0.05).
Demographic data and weight change in both groups are shown in Table 1.Weight change over time in both groups is shown in Figure 1.The changes in the other parameters were not found significantly different (Table 2).
Table 2: Baseline data in the 2 study groups.
| Group 1 | Group 2 | P | |
| Weight (kg) | 77.9 ± 13.1 | 84.3 ± 12.0 | 0.027 |
| Height (cm) | 160.3 ± 8.5 | 159.8 ± 7.7 | NS |
| BMI (kg m¯²) | 30.3 ± 4.7 | 33.2 ± 5.3 | 0.043 |
| Waist circumference (cm) | 102.0 ± 12.3 | 109.3 ± 10.4 | NS |
| Fasting blood sugar (mg dL¯¹) | 107.4 ± 7.4 | 110.8 ± 7.9 | NS |
| Postprandial blood sugar (mg dL–1) | 104.5 ± 13.7 | 116.8 ± 21.8 | NS |
| Hemoglobin A1c (HbA1c) (%) | 5.9 ± 0.3 | 6.0 ± 0.4 | NS |
| Fructosamine (µmol L–1) | 243.5 ± 20.9 | 232.3 ± 19.9 | NS |
| Alanine Aminotransferase (ALT) (U L–1) | 21.8 ± 8.6 | 24.3 ± 11.7 | NS |
| Gamma-GlutamylTransferase (GGT) (U L–1) | 17.9 ± 8.44 | 20.6 ± 12.5 | NS |
| Creatinine (mg dL–1) | 0.7 ± 0.1 | 0.7 ± 0.1 | NS |
| direct LDL cholesterol (mg dL–1) | 129.7 ± 32.2 | 131.0 ± 36.3 | NS |
| HDL cholesterol (mg dL–1) | 50.8 ± 17.0 | 53.0 ± 12.8 | NS |
| Triglyceride (mg dL–1) | 140.6 ± 82.5 | 134.5 ± 61.7 | NS |
| Insulin (µU mL–1) | 18.4 ± 35.9 | 13.5 ± 4.8 | NS |
NS: Not significant (P > 0.05).
DISCUSSION
Glucose homeostasis depends on the complex interaction between hormones, including insulin and amylin, which are produced by pancreatic beta cells, glucagon, which is produced by pancreatic alpha cells, and such gastrointestinal peptides as GLP-1 and GIP [9]. Abnormal regulation of these substances can lead to the clinical presentation of diabetes. In type 2 DM incretin axis abnormalities were reported to play an important role in progressive beta cell insufficiency [5]. Recent experimental trials performed in healthy individuals using artificial sweeteners along with glucose reported an increase in GLP-1 secretion, and suggest it might occur via the taste receptors in L cells [6,8]. Based on earlier short-term sweetener studies, the present study aimed to determine if aspartame a non-caloric sweetener taken regularly with food has a metabolic effect in prediabetic patients.
Brown [8] performed 75-g OGTT in 22 healthy volunteers, and glucose, insulin, and GLP-1 levels were measured for 180 min. The researchers reported that artificial sweeteners interact synergistically with glucose, increasing GLP-1 secretion. This increase in GLP-1 secretion was thought to be mediated by the stimulating effect of artificial sweeteners on the taste receptors in L cells. It was emphasized that patients with metabolic abnormalities, such as type 2 DM, related to the loss of incretin effect should be included. It was also pointed out that when the high number of people using artificial sweeteners daily is taken into account, investigating the effects of this substance on metabolism and body weight is of critical importance. They concluded that artificial sweeteners given in combination with glucose increased GLP-1 secretion, the clinical importance of which is yet to be determined.
Caniet [10] reported that 4 weeks of treatment with the indigestible sugar oligofructose increased GLP-1 secretion and had an antidiabetic effect in diabetic mice fed an extremely fatty diet. Based on their findings, they posited that instead of short-term treatment with sweeteners with the aim of improving glucose homeostasis via an increase in incretin secretion, longer treatment might be required. In the present study aspartame had no effect on the metabolic status of prediabetic patients, in terms of fasting and postprandial blood sugar, HbA1c, and blood glucose homeostasis, which is similar to the findings of the sweetener studies mentioned below.
Ma [11] loaded healthy volunteers with various levels of sucralose to investigate its effects on gastric emptying, and GLP-1, GIP, insulin, and blood glucose concentrations. They observed that sucralose given via intragastric infusion did not stimulate GLP-1 or GIP secretion, and did not delay gastric emptying. They also suggested that artificial sweeteners, apart from being substances that can be used instead of carbohydrates, have no therapeutic benefit in the control of diabetes. These findings are in accordance with those of other studies that reported sucralose does not affect plasma glucose or serum C-peptide levels in type 1 and 2 DM patients [12], and that stevioside a non-caloric sweetener does not stimulate GLP-1 or GIP in patients with type 2 DM [13]. Similarly, another study reported that during a 3-month period sucralose had no effect on fasting blood sugar or glycosylated hemoglobin levels in patients with type 2 DM [14]. Nonetheless, one clinical study reported that oral stevioside had no effect on GIP or GLP-1 secretion in patients with type 2 diabetes [13].
Horwitzet [15] investigated the effect of a single dose of aspartame and saccharine on glucose homeostasis in 12 healthy controls and 10 patients with type 2 DM via measurement of plasma glucose, insulin, and glucagon levels. The experimental group consisted of randomized participants given 400 mg of aspartame, 135 mg of saccharine, or no sweetener in order in weekly intervals; the aspartame dose was adjusted to 5-7 mg kg– 1 . Plasma samples were obtained 15, 30, 45, 60, 75, 90, 120, and 180 min after administration, and plasma insulin, glucose, and glucagon levels did not differ. Following aspartame consumption, although the area under the plasma insulin curve was high, it was not statistically significant, indicating that aspartame and saccharine did not affect blood glucose homeostasis in normal controls or patients with diabetes, which is in accordance with other similar studies on diabetic patients [16]. Colagiuri [16] compared the effects of adding aspartame and sucrose to the daily diet in patients with well-controlled non-insulin-dependent DM. In their double-blinded 6-week crossover study patients were given 45 g d–1 of sucrose (10g with each meal and 5g with each snack) during the sucrose treatment phase and 162 mg d–1 of aspartame as a sweetener equivalent (administered as sucrose was) during the aspartame treatment phase. Sucrose did not have a deleterious effect on glycemic control, glucose tolerance, or insulin activity, and furthermore, no difference between sucrose and aspartame was observed. Sucrose added to the diabetic diet did not negatively affect metabolic control in patients with well-controlled DM. Aspartame was shown to be a substitute for sugar in diabetic individuals; however, sucrose did not exhibit any advantage.
Hall [17] assumed that increased satiety with aspartame was demonstrated before and aimed to investigate the possible role of the satiety hormones CCK and GLP-1 on this effect. They additionally investigated the effects of aspartame’s ingredients phenylalanine and aspartic acid. They reported that increased satiety with aspartame was not associated with CCK- or GLP-1- mediated mechanisms, but that small changes in the circulating phenylalanine concentration could affect appetite. These findings showed that use of aspartame or amino acids at the doses studied did not increase CCK or GLP-1 secretion, or delay gastric emptying. As such, the researchers concluded that the satiety effects of aspartame might be influenced by factors other than the post-absorption factors defined here.
In the present study aspartame did not affect blood glucose or HbA1c, which supports the notion that when artificial sweeteners are given together with glucose they increase GLP-1 secretion via taste receptors; however, this finding does not mean that sweeteners do not stimulate GLP-1 secretion. As previously reported, some quantity of gustducin and GIP are known to reside together [7,6]; however, most K cells that express GIP were reported not to concurrently express α-gustducin. α-Gustducin was expressed in 15% of cells expressing GLP-1 [18]. Lastly, the potential stimulators of GLP-1 secretion remain unknown. Moreover, the limited localization of α-gustducin in incretin-expressing cells indicates that this signaling pathway does not play an important role in the regulation of GIP or GLP-1 secretion as a result of “getting the taste of” food [19].
Experiments performed on both cell lines and isolated primary cells indicate that K cells can sense glucose directly [20,21]; however, it is possible that these messages are transmitted by adjacent glucose-carrying mucosa cells [22]. In addition to glucose, fat is an efficient GIP and GLP-1 secretor. CD36 mediates the sensing of fats by the tongue. Nevertheless, it is not yet clearly understood how CD36 supports fatty acid uptake and signal transmission; as such, the sense of taste of both sugars and fats might be different from that in enteroendocrine cells [23]. In addition, there are important differences between species in terms of incretin hormone secretion. For instance, in rats [24] and humans [25] fructose stimulates GLP-1 secretion, but not in dogs [26].
Based on the available data, there appears to be high probability that the effect of artificial sweeteners on incretin secretion by enteroendocrine cells occurs via receptors other than taste. Nonetheless, the present study evaluated the effects of aspartame on direct LDL cholesterol, HDL cholesterol, and triglyceride levels, and the findings indicate that aspartame had no effect on lipid levels, as reported earlier [16,27]. Strategies to reverse the ongoing increase in the obesity rate should focus on decreasing energy intake and simultaneously increasing energy consumption. One method of decreasing energy intake is the consumption of low calorie foods, which can facilitate preserving ideal weight or weight loss. Use of strong artificial sweeteners instead of sugar has the potential to help people decrease the amount of energy intake without sacrificing taste.
The effect of aspartame on body weight has been studied. Blackburn [28] studied the effect of aspartame on weight loss and long-term weight control when added to an interdisciplinary weight control program. The study included 163 obese women; some were randomly assigned to consume food and beverages sweetened with aspartame and others were assigned to abstain from such foods during 16 weeks of a 19-week diet (active weight loss), a 1-year weight-preserving program, and 2 years of follow-up. Women in both groups lost 10% (10 kg) of their baseline body weight during active weight loss. In the aspartame group use of aspartame during active weight loss was significantly associated with the percentage of weight loss (r = 0.32, p< 0.001). During the weight preservation and follow-up periods the participants in the aspartame group gained 2.6% (2.6 kg) and 4.6% (4.6 kg) of their baseline weight after 71 and 175 weeks, respectively, versus 5.4% (5.4 kg) and 9.4% (9.4 kg), respectively, in the non-aspartame group. In the aspartame group the total amount of lost weight was higher (p=0.028) and less lost weight was regained during the weight preservation and follow-up periods (p=0.046), as compared to the non-aspartame group. These findings show that an interdisciplinary weight control program using aspartame can lead to long-term weight preservation following weight loss. These findings also suggest that including use of aspartame in a multidisciplinary weight loss program that also includes exercise and behavior modification could help control body weight in the long term. Blackburn [28] findings are similar to those of Porikos [29], who reported that the use of aspartame can facilitate weight control (when sugar was secretly replaced by aspartame a fast 25% decrease in energy intake occurred) [30,31], and to those of Evans [32].
A meta-analysis by De la Hunty [33] evaluated all studies on the effects of using aspartame only or some other strong artificial sweetener together with aspartame instead of sugar on energy intake and body weight. Randomized uncontrolled studies with healthy adults that did not measure energy intake for ≥24 h were not included in the analysis; as such, 16 studies were included. They reported that use of foods and beverages sweetened with aspartame instead of sugar resulted in significant decreases in both energy intake and body weight. The studies included in the meta-analysis had a mean investigation period of 12 weeks. This meta-analysis showed that use of foods and beverages sweetened with aspartame instead of sugar was an efficient way of preserving ideal weight and losing weight, without negatively affecting the taste of food. It was noted that aspartame did not increase calorie intake and, moreover, a significant decrease in energy intake was observed, as compared with the control groups. In summary, no evidence of a relationship between aspartame consumption and the development of obesity was observed. In contrast, when used as part of multidisciplinary weight control program aspartame facilitated long-term control of body weight; however, these data were obtained from short-term studies. It was concluded that in order to determine if tolerance to these effects occurs in the long term additional long-term investigations are required.
In the present study; the first group, diet was found effective on losing weight at the end of the third month. After aspartame was added, weight loss continued till the end of the 6 month. In the second group, weight loss was detected with aspartame and diet during the first three months. However; in the second three months, weight gain occurred after aspartame was discontinued. Both groups were compared regarding weight loss in the first and second period. Weight loss in the first group was greater than in the second group during second period (p=0.027). Similar BMI findings were observed. The changes in the other parameters were not found significantly different. As reported in earlier studies, these findings suggest that including aspartame in a multidisciplinary weight loss program consisting of exercise and behavior modification can help with long-term control of body weight. In addition, the weight loss obtained with aspartame can exert a long-term antidiabetic effect, as weight loss is one of the most important factors associated with the prevention of diabetes.
The DPP study investigated this matter in overweight or obese individuals with IFG or IGT, and reported that the risk of diabetes was decreased by 58% with intense life changes (intense life change targeting weight loss up to 7%) [34]. In the intense life style group analysis of the patients showed that among the 3 factors (weight loss, diet, and exercise) the prevention of diabetes was primarily associated with weight loss. For every 1 kg of weight loss the risk of diabetes decreased by 16% [35]. The Finnish Diabetes Prevention Study reported that in obese and overweight patients with IGT the risk of type 2 DM can be decreased by 58% with programmed weight loss and exercise [36]. BMI was observed to be a stronger determinant of type 2 DM than physical activity in a study performed in females [37].
The effect of aspartame on the mechanisms of weight loss can be explained, as follows: It can be assumed that the effect of aspartame on appetite, food intake, and weight control in motivated individuals is control of body weight via sweetening the taste of food without adding calories to the diet. Moreover, it can explain how low-energy sweeteners supported weight loss in their sensory-specific satiety (alternative food choices by pre-feeding a certain special type of food) [38,39]. Wooley [40] reported that use of artificial sweetener can decrease the kicking sweet cravings as effective as sugar itself and can lead to a decrease in energy consumption. Nonetheless, aspartame might have satisfied the high sensory responsibility in obese people trying to lose weight [41] and eliminated the craving of sweets, thusly leading to long-term weight preservation. It was reported that aspartame increases satiety [42-44]. It is also known that important satiety hormones in humans are CCK and GLP-1 [45- 49]. GLP-1 inhibits glucose release, delays gastric emptying, and suppresses appetite, thus leading to long-term weight loss [50]; it remains unclear if aspartame has similar effects.
In conclusion, aspartame has additional effect on weight loss in prediabetic patients. We need long term studies to investigate the relation between weight change and incretins.