Testosterone Deficiency and Erectile Dysfunction: A Practical Approach to Diagnosis and Management
- 1. Department of Urology, University of Queensland, Australia
- 2. Department of Environment, Earth & EcosystemsShore Street West Medical Centre, Australia
- 3. Department of Urology, St Andrew’s Pelvic Medicine Centre, St Andrew’s War Memorial Hospital, Australia
Abstract
Testosterone deficiency syndrome (TDS) is a clinical and biochemical syndrome frequently associated with age and co-morbidities and is characterized by deficiency in testosterone and relevant androgen-deficiency symptoms. The main physiological action of testosterone in male sexual function is in sexual desire by regulating the timing of the penile erectile with sex. However sexual dysfunction associated with TDS also includes erectile dysfunction (ED) and delayed ejaculation. The link between ED, testosterone deficiency and cardiovascular disorders is well documented. The recommended tests for men with ED include fasting glucose, cholesterol, lipids and testosterone level. Both ED and TDS are treatable conditions. A range of testosterone preparations are available for supplementation, and the combination of testosterone replacement therapy and phosphodiesterase type 5 inhibitors might improve outcomes in some cases. The selection of the testosterone replacement therapy should be a joint decision between an informed patient and his primary care physician, and regular follow-up should be conducted to assess treatment efficacy and surveillance for adverse events.
Keywords
• Testosterone deficiency
• Male hypogonadism
• Erectile dysfunction
• Male sexual function
Citation
Chung E, Al-Bermani OS, Fowler RP, Gillman MP (2013) Testosterone Deficiency and Erectile Dysfunction: A Practical Approach to Diagnosis and Management. J Endocrinol Diabetes Obes 1(2): 1012.
ABBREVIATIONS
TDS: Testosterone deficiency syndrome; ED: erectile dysfunction; PDE5: Phosphodiesterase inhibitor type 5
INTRODUCTION
Significant progress has been made in the field of medicine for erectile dysfunction (ED) since 1970s. In the past, male sexual dysfunction was thought to be purely psychogenic but increased understanding in the erectile physiology at molecular level has shown that testosterone deficiency plays a major role in sexual dysfunction. Men with low testosterone levels may often be overlooked as the association between testosterone deficiency syndrome (TDS) and its related co-morbidities such as cardiovascular disease and metabolic syndrome is not appreciated and at times, the symptoms and signs of TDS may not be obvious. This review article aims to provide primary care practitioners a practical approach to the diagnosis and management of testosterone deficiency in patients with ED.
Testosterone and ED
Testosterone is vital for normal functioning throughout a man’s life and a reduced testosterone level could compromise the man’s general well-being and his sexual function. Published literature showed that testosterone controls, directly or indirectly, several mechanisms pertinent to penile erectile function such as the promotion and differentiation of penile stem cells to penile smooth muscle cell phenotype, activity of cavernosal nitric oxide synthase and that of RhoA-kinase pathway [1]. More importantly, testosterone also regulates the expression of phosphodiesterase type 5 (PDE5), and in so doing maintains a homeostatic ratio between isoforms of nitric oxide synthase and PDE5 enzyme that is responsible for penile erection [2].
Apart from penile erection, testosterone also regulates other aspects of male sexual desire. While erections are possible in hypogonadal conditions, studies showed that patients with decreasing levels of sexual desire have progressively lower concentrations of testosterone than men who maintain their sexual desire. Sexual desire/libido is universally accepted as one of the most testosterone-dependent aspects of male sexual behaviors [3]. It appears that testosterone is responsible in the regulation of the timing of the erectile process as a function of sexual desire, thereby coordinating erection with sex [4]. Some studies showed that higher androgen level potentially play a dominant role in increased frequency of autoeroticism behaviors [5] and propensity for extramarital affairs [6]. Recent studies have also highlighted the role of testosterone in ejaculatory dysfunction via the effect of testosterone on nitric oxide metabolism in the central and peripheral control of ejaculation that could be accountable in condition such as premature ejaculation [7].
MATERIALS AND METHODS
Diagnosis of TDS
There is strong evidence that ED is an independent marker for subsequent cardiovascular disease and that the incidence of ED is more common in older patients with higher cardiovascular risks. The prevalence of low testosterone in men with ED has been estimated at 10-20% [8]. The association between ED, low testosterone level and cardiovascular disease are well recognized and these disorders are associated with the presence of metabolic syndrome [9]. In fact a clear negative relationship exists between the presence of the risk factors for metabolic syndrome and levels of circulating testosterone in patients with ED [9].
The clinical presentation of TDS, defined as both low testosterone level and clinically significant symptoms, can be at times nonspecific and often overlooked or under-diagnosed. The clinical symptoms associated with TDS can be divided into 3 main groups, namely psychosomatic, metabolic and sexual related problems. Unfortunately none of the symptoms is specific to the low androgen state but each symptom may raise the suspicion of TDS.
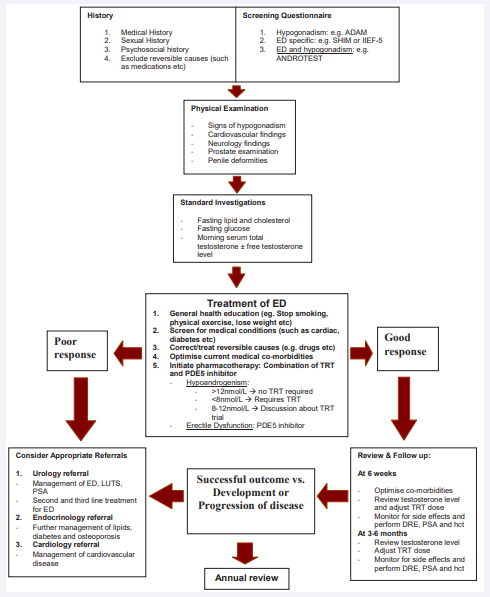
Patients with ED, together with other symptoms such as reduced libido, decreased muscle mass and strength, presence of type 2 diabetes mellitus and metabolic syndrome should be screened for TDS (Figure 1).
Figure 1: A practical algorithm for the management ED in suspected hypogonadal men. (ED- erectile dysfunction, ADAM- Androgen Deficiency in Aging Male, SHIMSexual Health Inventory in Male, IIEF- International Index of Erectile Function, T- testosterone, PDE- phosphodiesterase, LH- luteinising hormone, FSH- follicle stimulating hormone, DHEA- dihydroepiandrosterone, TSH- thyroid stimulating hormone, SHBG- sex hormone binding globulin, TRT- testosterone replacement therapy, SSRIselective serotonin reuptake inhibitor, TCA- tricyclics antidepressant, PSA- prostate specific antigen, DRE- digital rectal examination, hct- hematocrit)
The initial assessment of subjects with clinical suspicion of TDS should include a comprehensive evaluation of medical and psychosocial, associated co-morbidities as well as identification of any reversible factors and conditions that could impact on the prescription of testosterone replacement therapy (TRT) such as in subjects with undiagnosed prostate cancer, obstructive sleep apnea and congestive heart failure (Figure 1). While standardized questionnaires such as the Androgen Deficiency in Aging Male (ADAM) checklist is designed to identify symptoms and signs of TDS [10], they are not very specific but may play a role in encouraging men to discuss their symptoms and for monitoring changes in symptoms. Previously, a brief (12 items) structured interview called the ANDROTEST has been designed specifically to screen for hypogonadism in patients with sexual dysfunction [11].
Physical examination should focus on cognition, neurological, cardiovascular and urological findings. In men over the age of 40 years, prior to TRT, the risk of prostate cancer must be assessed and if the digital rectal examination of the prostate gland or prostate specific antigen (PSA) reading is abnormal, further urological assessment should be arranged [10]. The recommended tests for men with ED are fasting glucose, cholesterol, lipids and testosterone level.
Laboratory diagnosis of testosterone deficiency: The exact pathogenesis of low testosterone remains contentious and several proposed mechanisms include decreased Leydig cell function, age-related increased in sex hormone binding globulin (which binds testosterone and lowers free bioavailable testosterone level), blunted circadian steady state of testosterone and decreased luteinizing hormone (LH) pulse by the hypothalamus [12].
The biochemical diagnosis of TDS is based on the measurement of serum total testosterone (TT), preferably before 11 am, though the diurnal rhythm of testosterone is less marked in men aged over 40 years. Transient decrease in serum testosterone level such as in acute physical illness should be excluded by careful evaluation and repeat testosterone measurement. Symptoms of TDS usually present with TT levels below 8 nmol/l (230 ng/dl), but may occur at various TT levels between 8 and 12 nmol/l (230 to 350 ng/dl). As a general rule of thumb, the mean serum total testosterone decreases by 1% per year after the age of 40 years [13]. If the serum testosterone level is between 8 and 12 nmol/L, free testosterone (FT) level obtained by repeated measurements of TT with sex hormone binding globulin (SHBG) and albumin, or through equilibrium dialysis, may be helpful.
While theoretically serum FT is more representative of the biological activity of testosterone, these assays may be more difficult to carry out (especially equilibrium dialysis, the reference method for FT), inaccurate (FT assay by testosterone analogue and ‘Free Androgen Index’), or can only be performed by some laboratories. Furthermore there are no accepted lower limits of free testosterone for the diagnosis of TDS [10]. The FT assays are generally set aside for the repeat assay and to certify the significance of a borderline TT level, or when SHBG concentration may be altered.
In the case of low or borderline testosterone value, the assay should be repeated, because of the frequent intra-individual fluctuations of serum testosterone, unless physical evidence of hypogonadism (i.e. atrophied testes) is present. The assays of serum luteinizing hormone (LH) and follicle stimulating hormone (FSH) (to specify the primary/testicular or secondary/ hypothalamic or pituitary origin of hypogonadism), prolactin (to screen for hyperprolactinaemia), and possibly SHBG should be combined with the repeat testosterone assay.
Testosterone Replacement Therapy
Whilst there is no consensus on the lower limit of the normal range for testosterone, there is a general consensus that a total testosterone level above 12 nmol/L (350 ng/dl) does not warrant TRT [10]. Men with serum total testosterone levels below 8 nmol/L (230 ng/dl) will usually benefit from TRT. For men with borderline or low/normal TT (8-12 nmol/l or 230-350 ng/dl), several meta-analyses have established that significant improvements may be achieved with TRT especially with regards to body composition and sexual function [14]. The indication to start TRT must be based on complete clinical assessment with an evidence of hypoandrogenism.
When choosing which TRT to prescribe, primary care physician must exercises good clinical judgment together with adequate knowledge of the advantages and drawbacks of TRT and considers the bioavailability, safety, tolerability, efficacy and preference of each TRT product (Table 1).
Table 1: Various testosterone formulations.
| Delivery Method | Half-life | Strengths available | Adverse Event |
| SC Implant | 90 days | 100mg 200mg |
Twice per year administration. Placement is invasive with risk of extrusion and site infections |
| IM injection | 14-21 days | 100mg 250mg |
Pain at injection site. Fluctuation in testosterone level. |
| Transdermal Gel | 6 hours | 50mg in 5g sachet | Possible transfer during intimate contact |
| Transdermal solution | 6 hours | 30-120mg solution | Possible transfer during intimate contact |
| Transdermal Patches | 10 hours | 12.2mg (2.5mg/24hrs) 24.3mg (5mg/24hrs) |
Skin irritation |
| Cream | 6 hours | 10mg/g (1% w/w) 20mg/g (2% w/w) 50mg/g (5% w/w) |
Skin irritation |
| Depot Injection | 4-5 days | 250mg/1mL | Wild fluctuations in circulating testosterone levels Relative higher risk of polycythemia |
| Oral Capsule | 4 hours | 40mg | Variable clinical response First pass metabolism; must be taken with meals |
| IM Injection | 34 days | 1000mg/4mL | Pain at injection site |
Abbreviations: IM= Intra Muscular
Hypogonadal men restored to eugonadal state with TRT will experience improvement in sexual functions, particularly erectile, ejaculation, orgasm and penile sensations; and restored or enhanced responsiveness to PDE5 inhibitors [10]. The contraindications and cautions for the use of testosterone remain controversial. Men with severe lower urinary tract symptoms, polycythaemia (hematocrit >50%), untreated obstructive sleep apnoea, severe congestive cardiac failure, breast or prostate cancer should not be started on TRT without appropriate assessment and treatment by the respective specialists. The use of TRT in clinically symptomatic hypoandrogenism men treated previously for localized prostate cancer remains controversial [15]. The selection of the TRT preparation should be a joint decision between an informed patient and his physician. It is important to counsel younger men who wish to father children that exogenous TRT paradoxically results in infertility and this could potentially be irreversible.
The effects of TRT may be perceived within 2-4 weeks, but sexual effects may sometimes take 3-6 months to become apparent and even up to 1 year for the nocturnal erections to reach normal range in previously untreated hypogonadal patients [16]. Some studies showed that TRT resulted in a significant but moderate improvement in all aspects of sexual function in men with testosterone at baseline (12 nmol/ml or 350 ng/dl) [17]. The magnitude of the effect on erectile function is inversely related to baseline concentration of testosterone.
Since the occurrence of any adverse events during therapy (such as an elevated hematocrit or PSA) requires rapid discontinuation of TRT, short-acting preparation is preferred over long-acting depot preparation in the initial treatment of hypogonadal men, but there is no contraindication to start with longer acting preparations. At present the use of gonadotophic hormones such as human chorionic gonadotropin and selective estrogen receptor modulator (such as clomifene citrate) is not recommended except in selected cases of male infertility [18].
Regular patient follow up is paramount following TRT initiation (Figure 1). Sustained supraphysiological levels of testosterone should be avoided. Failure to benefit after adequate testosterone treatment for 3 to 6 months should result in further investigation to rule out associated comorbidities and/ or discontinuation of TRT. Patients should be monitored at 3 months initially, and later at 3 to 6 monthly follow-up for adjustment of TRT and surveillance for any complication. Once a steady testosterone state is achieved, annual follow-up can be instituted.
Testosterone & PDE5 Inhibitors in ED: As previously mentioned, testosterone modulates the expression of isoforms of nitric oxide synthase and PDE5 enzyme [19]. Several studies have demonstrated that TDS is associated with a reduced PDE5 inhibitors efficacy [19]. This underlies the important concept that TDS must be ruled out, or if present, should be adequately treated, before PDE5 inhbitors are prescribed in men with ED. Published literature showed the combination of TRT and PDE5 inhibitors enhances the overall efficacy in men with were previously PDE5 inhibitors unresponsive [19]. Longer duration of combination TRT and PDE5 inhibitors use appeares to increase the patient response rate to PDE5 inhibitors [20].
At present controversies exist whether men with hypoandrogenism and ED should be treated initially with PDE5 inhibitors, TRT or in combination [16]. While TRT should be considered as the first line treatment in patient with ED who have hypoandrogenism, this approach might not be adequate and/or appropriate as a monotherapy in all cases because of the multifactorial nature of ED. Furthermore, some studies have suggested that erectile response may actually decreased when higher levels of testosterone were reached in men who were not hypogonadal [21]. Hence, further studies are needed to evaluate the benefits of combination TRT and PDE5 inhibitors.
CONCLUSIONS
Mild hypogonadism/hypoandrogenism is not uncommon and the combination of symptoms and signs can assist in the identification of TDS. A biochemical confirmation of TDS is mandatory prior to TRT. Although hypoandrogenism can be the main cause of ED in younger patients, ED is often multi-factorial in pathophysiology and therefore it is unlikely that TDS is the sole contributor for the development and progression of ED. For this reason, combination therapy using TRT and PDE5 inhibitors should be considered as first line in the majority of cases as it might improve the clinical outcome better than TRT only.