Effects of BMI on Non-Surgical Management Outcomes in SIJ Pain Patients: A Retrospective Cohort Study
- 1. The Department of Orthopedic Surgery, University of Minnesota, USA
- 2. University of Minnesota Medical School, USA
Abstract
Objective: Sacroiliac joint (SIJ) dysfunction is typically managed through nonsurgical approaches or surgical intervention, such as SIJ fusion. Many insurance providers use body mass index (BMI) as a criterion for surgical approval, with a common cutoff set at 35 kg/m². This practice often restricts obese patients to nonsurgical management options. However, there is limited evidence regarding the outcomes of nonsurgical management for SIJ pain in individuals with varying BMI categories.
Design: Retrospective Review
Setting: Academic medical institution
Participants: Adult patients ≥ 21 years of age who received nonsurgical treatment for sacroiliac joint dysfunction between 2021 and 2023. Participants were classified using the National Institutes for Health body mass index (BMI). Patients with a BMI of 30 to 39 with no significant comorbidity are considered obese, patients with a BMI of 35 to 39 with a significant comorbidity or a BMI of 40 or greater are considered morbidly obese.
Interventions: Non-surgical management (physical therapy, injection, radiofrequency ablation, bariatric surgery, pain management), SIJ fusion
Main Outcome Measure(s): All subjects completed the Visual Analog Scale (VAS) and Oswestry Disability Index (ODI) at baseline and 12 months.
Results: Overall, mean VAS demonstrated negligible improvement at 12 months (1.8-point improvement; p=0.77). Over the 12-month follow-up period, BMI category did not impact mean improvement in VAS (ANOVA p=0.99). Mean ODI at 12 months demonstrated negligible improvement (9.3-point improvement; p=0.50). BMI category did impact mean improvement in ODI (ANOVA p=0.54).
Conclusions: Our findings suggest the success of nonsurgical management of SIJ pain is limited and difficult to predict which is consistent with current literature as patients treated with continual nonsurgical management demonstrate no long-term improvement in pain or disability.
KEYWORDS
- SI joint
- Pain
- Disability
- Physical therapy
- Injection
- Fusion
- BMI
CITATION
Mutyaba I, Reutzel B, Beckmann M, Odland K, Polly Jr, DW (2025) Effects of BMI on Non-Surgical Management Outcomes in SIJ Pain Pa- tients: A Retrospective Cohort Study. J Family Med Community Health 12(1): 1207.
INTRODUCTION
The sacroiliac joint (SIJ) contributes to 15-30% of all chronic low back pain (LBP) [1-4].The causes of SIJ pain are complicated and not completely understood, encompassing factors like joint mobility, load transfer issues, and links to conditions such as osteoarthritis or spondyloarthropathies. Patients with SIJ pain have decreased quality of life with levels similar to other common surgically treated spine conditions. The burden of disease associated with SIJ pain is at least as high as that associated with other musculoskeletal conditions such as hip osteoarthritis, degenerative spondylolisthesis or spinal stenosis, conditions that are often treated surgically [5,6].
In the United States, the prevalence of obesity (defined as BMI>30) has been increasing, from 30.5% in 2000 to 42.4% in 2018 [7,8]. Furthermore, 70% of people aged 60 and older are estimated to be overweight or obese [9- 13]. As the population both ages, and the proportion of those with obesity increases, there is a growing need to address SIJ pain in obese individuals. Prior studies have associated obesity with musculoskeletal issues, lumbar disc degeneration, low back pain, and indicates that a BMI greater than 25 kg/m² raisesthe risk oflumbardegenerative disease and leads to poorer surgical outcomes. However, there is currently no published evidence to indicate that obesity is a risk factor for developing sacroiliac joint (SIJ) pain.
SIJ pain management may be treated surgically or non- surgically. Initial nonsurgical management (NSM) may include conservative measures such as physical therapy, chiropractic care, or medical management [14,15]. If pain cannot be managed through these interventions, other treatments such as intra-articular injections and radiofrequency ablation are often employed. Previous studies have indicated that obese patients, particularly those with a BMI between 35 and 40, experience equal or even greater improvements with SIJ fusion compared to their non-obese counterparts. However, a majority of insurance carriers and healthcare systems use BMI as a criterion to approve surgery, with 35kg/m2 as the most common BMI cutoff [16]. As the population continues to age and obesity rates rise, an increasing number of patients with SIJ pain may be denied surgery due to BMI, limiting them to continue NSM longer than their non-obese counterparts. Currently, there is no evidence surrounding the outcomes of NSM for SIJ pain in relation to BMI.
The purpose of the current study was to examine data from a single institution to examine the impact of obesity on patient-reported outcomes after nonsurgical SIJ pain management.
METHODS
Case Ascertainment
Data was retrospectively collected from patients diagnosed with sacroiliitis based on >3/5 positive provocative SIJ maneuvers on physical exam and 2 separate image guided SI joint injections with >75% improvement at a single institution between 2021 and 2023. Patients eligible for analysis (n=256) were categorized into National Institutes of Health (NIH) BMI classes by preoperative weight. The BMI classes were defined as: <30 kg/m2 overweight, 30.0 to 34.9 kg/m2 obesity class I, 35.0 to 39.9 kg/m2 obesity class II, and ≥40.0 kg/m2 obesity class III.
Patient Population
Eligibility criteria included adult patients between the ages of 21 and 80 with a confirmed diagnosis of SIJ pain due to degenerative sacroiliitis and/or sacroiliac joint disruption established from typical historical findings (pain in the back below L5, buttocks or legs, including a positive Fortin finger test), and SIJ pain elicited on at least 3 of 5 established physical examination provocative tests which include the FABER (Flexion, Abduction and External Rotation), thigh thrust, Gaenslen’s, compression and distraction tests17. Patients were excluded if they had other back conditions that caused moderate-to-severe pain, other sacroiliac pathologies (e.g., autoimmune sacroiliitis, tumor, infection, fracture), known osteoporosis, chronic rheumatologic disease or other conditions.
Intervention
All patients received one or more of the following NSM treatments to manage sacroiliac joint dysfunction and pain: physical therapy (PT) and aquatic therapy (AT) of various duration and frequency following American Physical Therapy Association (APTA) guidelines, intraarticular SIJ steroid injections, radiofrequency (RF) ablation of lateral branches of the sacral nerve roots, pain clinic referral, and bariatric surgery. Many also received different forms and dosages of oral pain control medicines such as opioids, nonsteroidal anti-inflammatory drugs (NSAIDs) and acetaminophen.
Follow-Up
Outcome measures on pain and disability were collected at baseline and at patient follow-up visits at 6 weeks, 3 months, 6 months and 12 months. Dysfunction due to pain was assessed using Oswestry Disability Index (ODI), a patient-completed questionnaire that is validated [16], and commonly used to address dysfunction due to back pain [18]. SI joint pain was assessed using a 0-10 visual analog scale (VAS). The minimum clinically important difference (MCID) represents the smallest change in a patient-reported outcome measure that is of genuine clinical value to patients [19], and the values used for MCID in the current study was 13 for ODI [20], and 20 for VAS.
Statistical Analysis
Analysis focused on patients receiving the different non-surgical management interventions for sacroiliac joint dysfunction. Body mass index at baseline was classified as normal or overweight (BMI < 30), Class 1 obesity (BMI 30- <35), and Class 2 obesity (BMI 35 - <40). Patient reported outcomes as well as changes from baseline, were compared by BMI class using one-way Analysis of Variance (ANOVA) with post hoc Bonferroni pairwise comparisons at each time interval. MCID was examined by applying previously published distribution-based methods. All statistical analyses were performed using SPSS version 28.0 (IBM Corporation, Armonk, NY). P values <.05 were considered statistically significant.
RESULTS
Baseline Characteristics
A total of 149 patients (110 females; 39 males) were included (Figure 1).
Figure 1: CONSORT flow diagram.
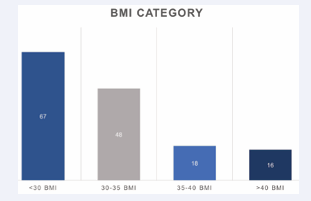
The mean BMI for the cohort was 30.1 (±6.3). Amongst the respective BMI groups, patients with a <30 BMI comprised the majority of the cohort (45%), compared to the 30-35 BMI group (32.2%), the 35-40 BMI group (12.1%), and the >40 BMI group (10.7%) (Figure 2).
Figure 2: BMI Category.
Among non-surgical interventions; 44% were joint injections, 40% attended physical therapy, 7% were pain clinic referrals, 5% underwent bariatric surgery, 2% received radiofrequency ablation, and 1% participated in aquatic therapy. The mean baseline pain (VAS) score was 6.2 (±2.3), with 3 patients reporting no pain (VAS score 0), 19 reporting mild pain (1-3), 48 moderate pain (4-6), and 73 severe pain (7-10). The mean baseline disability score (ODI) was 54.3 (±16.3) with 4 patients reporting minimal disability (ODI 0-20), 27 with moderate disability (21-40), 60 Severe (41-60), 52 crippled (61-80) and 6 reporting bedbound level of disability (81-100) (Table 1).
Table 1: Patient Characteristics for Sacroiliac Joint (SIJ) Non-Surgical Management Cohort
|
|
Frequency (n) |
Percent (%) |
Mean (±SD) |
|
Age |
|
|
56.3 (±13.7) |
|
Sex Male Female |
39 110 |
25% 75% |
|
|
|
|||
|
Intervention Injection Physical Therapy Pain Clinic Radiofrequency Ablation Aquatic Therapy Bariatric Surgery |
66 60 11 3 2 7 |
44.2% 40.2% 7.3% 2.0% 1.3% 4.7% |
|
|
|
|||
|
BMI <30 30-35 35-40 >40 |
67 48 18 16 |
45.0% 32.2% 12.1% 10.7% |
30.1 (±6.3) |
|
VAS 0 None 1-3 Mild 4-6 Moderate 7-10 Severe |
3 19 48 73 |
2.0% 12.8% 32.2% 49.0% |
6.2 (±2.3) |
|
ODI 0-20 Minimal 21-40 Moderate 41-60 Severe 61-80 Crippled 81-100 Bedbound |
4 27 60 52 6 |
2.7% 18.1% 40.3% 34.9% 4.0% |
54.3 (±16.3) |
|
Total |
149 |
100 |
|
Primary Outcomes for all BMI Categories
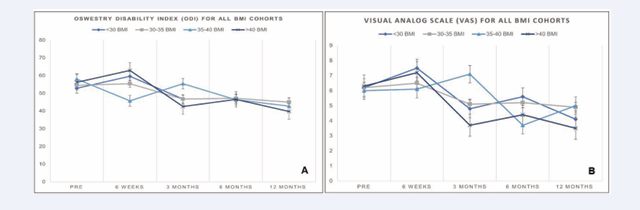
Overall, the mean VAS pain score improved from 7.1 at baseline to 5.3 at 12 months, signaling a 1.8-point improvement (p=0.77). BMI category did not impact mean improvement in VAS score (one-way ANOVA; p=0.99). The mean ODI score improved from 51.0 at baseline to 41 at 12 months, signaling a 9.3-point improvement (p=0.50). BMI category did not impact mean improvement in ODI score (one-way ANOVA; p=0.54) (Tables 2 & 3; Figures 3a & 3b).
Table 2: Primary Outcomes for Entire SIJ Non-Surgical Management Cohort (n=149).
|
|
Pre |
6 Weeks Post |
3 Months Post |
6 Months Post |
12 Months Post |
Mean Change (SD) |
MCID |
p value |
|
VAS |
7.1 |
7.2 |
5.5 |
5.8 |
5.3 |
1.8 |
2 |
0.77 |
|
ODI |
51.0 |
49.0 |
48.0 |
41.7 |
41.7 |
9.3 |
13 |
0.50 |
|
Repeated Measures ANOVA; VAS = Visual Analog Scale; ODI = Oswestry Disability Index; MCID = Minimal Clinically Important Distance |
||||||||
Table 3: VAS and ODI Mean Change from Baseline to 12 Months Between BMI Categories.
|
|
BMI <30 (n=67) |
BMI 30-35 (n=48) |
BMI 35-40 (n=18) |
BMI >40 (n=16) |
MCID |
p value |
|
VAS Mean Change |
1.6 |
2.0‡ |
1.7 |
2.5‡ |
2 |
0.99 |
|
|
|
|
|
|
|
|
|
ODI Mean Change |
13.0‡ |
12.3 |
21.2‡ |
9.0 |
13 |
0.54 |
|
One-way ANOVA; ‡ = Met MCID; MCID = Minimal Clinically Important Difference |
||||||
Figure 3 a: Visual Analog Scale Scores for All BMI Categories between SIJ Fusion Cohort and NSM Cohorts. b. Oswestry Disability Index Scores for All BMI Categories Between SIJ Fusion and NSM Cohorts.
Primary Outcomes for BMI <30 Cohort
In the <30 BMI cohort, the mean VAS pain score improved from 5.6 at baseline to 3.9 at 12-month follow- up, indicating only a 1.6-point improvement from baseline (p=.003). However, MCID was achieved by 16 patients (23.8%) at the 12-month follow-up (Table 4).
Table 4: Primary Outcomes for <30 BMI Cohort (n=67).
|
|
Pre |
12 Months Post |
Overall Mean Change (SD) |
MCID |
p value |
|
VAS |
5.6 |
3.9 |
1.6 |
2 |
.003 |
|
ODI |
49.0 |
36.0 |
13.0‡ |
13 |
<.001 |
|
Paired Samples T-test; VAS = Visual Analog Scale; ODI = Oswestry Disability Index; MCID = Minimal Clinically Important Difference; ‡ = Met MCID |
|||||
The mean ODI score improved from 49.0 at baseline to 36.0 over the same time period, indicating a 13-point improvement (p<.001). MCID was achieved by 15 (22.3%) patients at the 12-month follow-up.
Primary Outcomes for 30-35 BMI Cohort
In the 30-35 BMI cohort, the mean VAS pain score improved from 7.0 at baseline to 5.0 at 12-month follow- up, indicating only a 2-point improvement from baseline (p=.002). MCID was achieved by only 10 patients (20.8%) at the 12-month follow-up. The mean ODI score improved from 60.7 to 48.4 over the same time period, indicating a 12.3-point improvement (p=.006). MCID was achieved by 7 (14.5%) patients at the 12-month follow-up (Table 5).
Table 5: Primary Outcomes for 30-35 BMI Cohort (n=48).
|
|
Pre |
12 Months Post |
Overall Mean Change (SD) |
MCID |
p value |
|
VAS |
7.0 |
5.0 |
2.0‡ |
2 |
.002 |
|
ODI |
60.7 |
48.4 |
12.3 |
13 |
.006 |
|
Paired Samples T-test; VAS = Visual Analog Scale; ODI = Oswestry Disability Index; MCID = Minimal Clinically Important Difference; ‡ = Met MCID |
|||||
Primary Outcomes for 35-40 BMI Cohort
In the BMI 35-40 cohort, the mean VAS pain score improved from 6.1 to 4.4 at 12-month follow-up, indicating only a 1.7-point improvement (p=0.07). MCID was achieved by only 3 patients (16.6%) at the 12-month follow-up. The mean ODI score improved from 58.0 to 36.8 at 12-month follow-up, indicating a moderate 21.2-point improvement (p=0.01). MCID was achieved 7 patients (38.8%) at the 12-month follow-up (Table 6).
Table 6: Primary Outcomes for 35-40 BMI Cohort (n=18).
|
|
Pre |
12 Months Post |
Overall Mean Change (SD) |
MCID |
p value |
|
VAS |
6.1 |
4.4 |
1.7 |
2 |
0.07 |
|
ODI |
58.0 |
36.8 |
21.2‡ |
13 |
0.01 |
|
Paired Samples T-test; VAS = Visual Analog Scale; ODI = Oswestry Disability Index; MCID = Minimal Clinically Important Difference; ‡ = Met MCID |
|||||
Primary Outcomes for >40 BMI Cohort
In the BMI >40 cohort, the mean VAS pain score improved from 8.0 at baseline to 5.5, indicating a 2.5-point improvement (p=0.12). MCID was achieved by only 2 patients (12.5%) at the follow-up. The mean ODI score improved from 47.5 to 38.5, indicating a 9.0-point improvement (p= 0.8). MCID was achieved by only 1 patient (6.2%) at the 12-month follow-up (Table 7).
Table 7: Primary Outcomes for >40 BMI Cohort (n=16).
|
|
Pre |
12 Months Post |
Overall Mean Change (SD) |
MCID |
p value |
|
VAS |
8.0 |
5.5 |
2.5 |
2 |
0.12 |
|
ODI |
47.5 |
38.5 |
9.0 |
13 |
0.80 |
|
Paired Samples T-test; VAS = Visual Analog Scale; ODI = Oswestry Disability Index; MCID = Minimal Clinically Important Difference; ‡ = Met MCID |
|||||
Comparison of Primary Outcomes between SIJ Fusion and NSM across BMI Categories
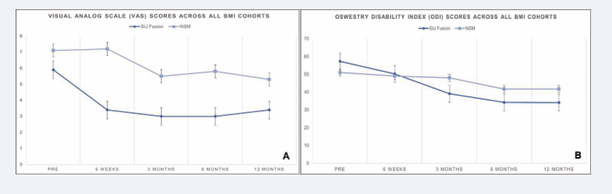
In our NSM group, mean SIJ pain improved from 7.1 to 5.3 at 12 months (1.8-point improvement, p=0.77) versus our previously reported SIJ fusion cohort [21], where the mean SIJ pain score improved from 5.9 at baseline to 3.4 at 12 months (2.5-point improvement, p<.0001). Over the 12-month follow-up period, BMI category did not impact mean improvement in SIJ pain score for the NSM group (one-way ANOVA p= 0.99) nor did it impact mean improvement in SIJ pain score for our previously reported SIJ fusion cohort21 (one-way ANOVA p= 0.08) (Figures 4a & 4b).
Figure 4 a: Oswestry Disability Index Scores for All BMI Categories. b. Visual Analog Scale Scores for All BMI Categories.
In our NSM group, mean SIJ disability score improved from 51.0 at baseline to 41.6 at 12 months (9.3-point improvement, p= 0.50) versus our previously reported SIJ fusion cohort [21], where the mean SIJ disability score improved from 57.3 to 34.1 at 12 months (23.2-point improvement, p<.001). Over the 12-month follow-up period, BMI category did not impact mean improvement in SIJ disability score for the NSM group (one-way ANOVA p=0.54) whereas BMI category did impact mean improvement in SIJ disability scale score for our previously reported SIJ fusion cohort [21], (one-way ANOVA p=0.03). Combining all time points up to month 12, the improvement in VAS SIJ improvement was only 0.7 points greater for our previously reported SIJ fusion group [21], compared to our NSM group (p=0.10) Combining all time points up to month 12, the improvement in ODI SIJ improvement was 13.9 points greater for our previously reported SIJ fusion group [21], compared to our NSM group (p=0.39).
DISCUSSION
Prior to undergoing surgical intervention for SIJ pain, NSM is initiated in an attempt to improve pain and function and potentially further delay or avoid surgery. A wide range of interventions to manage SIJ pain are used however, many lack evidence of clinically relevant long- term effects.
Intraarticular Injections
SIJ injections with local anesthetic and steroid for therapeutic purposes can be considered if SIJ pain continues despite physical therapy (PT) [22]. Injections were the most commonly utilized intervention in our cohort with 44% receiving at least one injection. Intraarticular injections, specifically those using some type of image- guidance have been investigated extensively suggesting they are an effective treatment modality in reducing pain [22-28]. The duration of pain improvement varies slightly with an average duration of improvement ranging from 8-10 months in studies that followed patients over a longer follow-up period [23]. Two blinded trials from Luukkainen et al., suggest short-term effectiveness for periarticular steroid injection (3mL of steroid and local anesthetic (p=0.047; p=0.02, respectively) [22,23]. The only study [29], evaluating bilateral intraarticular SIJ injection using fluoroscopic guidance reported greater than 70% relief at 1 month in 5/6 who received steroid (1.5mL of cortivazol) vs 0/7 who received placebo (1.5mL saline), at 3 months and 6 months respectively. Schmidt et al. [30], found the efficacy of injections was low in patients with a history of lumbar or lumbosacral fusion; however, nearly two thirds of patients noted >50% pain reduction for 6 weeks with a mean duration of relief for approximately 8 months. Previous studies [31,32], have reported peri-articular SIJ injections were more effective (81% and 100%, respectively) than intra-articular SIJ injections in most patients with SIJ-related pain. However, the proportion of effectiveness between the two injection types in patients with pain specifically at or around the PSIS, characteristic of this disorder, remains unclear.
Physical Therapy
There have been multiple studies investigating the efficacy of physical therapy for SIJ pain, many of which focus on pregnant women given the high prevalence of SIJ pain in that population with an estimated prevalence of 49% [33], albeit not the patient population we investigated. In our cohort, 40.2% participated in physical therapy which was the second most frequently utilized NSM intervention for SIJ pain. Nilsson-Wikmar et al. [33], conducted a randomized trial among 3 treatment groups: information and elastic belt, home-exercise program, and in-clinic exercise; they found that all 3 groups improved from 38 weeks gestation to 12 months post-partum and there was no clinically significant difference across groups. Delitto et al. [34], had a similar result after 8 weeks of physical therapy, again examining low back pain in post- partum women who were assigned to 1 of 3 treatment protocols: a group that performed exercises to increase the force of the diagonal trunk muscle systems, a group that received training of the longitudinal trunk muscle systems, and a group that was instructed to refrain from exercises. This may signify that physical therapy does not play a significant role in improving SIJ pain and that some other factor is at play as all groups ultimately did see improvement. In regard to aquatic therapy for SIJ pain, there have not been any studies to the authors’ knowledge that have investigated this modality. The theoretical benefit of aquatic therapy being that an aquatic environment provides less axial load and therefore less pain when performing exercises geared toward improving muscle imbalances and SIJ kinematics. The benefit of this type of physical therapy is hypothesized to most benefit patients with significant pain or large BMI limiting participation in land-based physical therapy.
Radiofrequency Ablation
Radiofrequency ablation (RFA) can be considered for the patient who has repeated return of significant sacralgia pain despite technically adequate SI joint injection. RFA is a treatment that was utilized by a subset of our patients presenting with SIJ pain. This involves selective ablation and denervation of the nerves innervating the SIJ. SI joint RFA is technically difficult to perform largely due to patient anatomic variability and technique differences between providers and is typically associated with short-term pain relief [35,36]. Two blinded trials suggest short-term effectiveness for RFA of sacral nerve roots [37]. Return of pain 6 to 12 months following RFA is common secondary to regeneration of nerve innervation. Long-term relief of months to approximately a year has been reported in approximately 1/3 of patients [38,39]. A meta-analysis by Aydin et al. pooled data from 10 studies and their results demonstrated a greater than 50% improvement in pain at 3 months and 6 months, however, the typical limitations of meta-analysis were present such as heterogeneity, variations in location, technique and type of RFA utilized [40]. Prolotherapy and radiofrequency ablation may offer a potential benefit as therapeutic modalities, although limited data support their use as a primary treatment modality. Limitations in effectiveness are due to the complex innervation of the SIJ [41]. At the time of this writing, limited evidence in controlled studies was available to judge the efficacy. Overall, the efficacy of RFA is limited by the inability to denervate the anterior neural structures to the SIJ and by regeneration of nerves over a several-month period. SI joint RFA is usually not a curative procedure due to nerve regrowth with return of pain.
Bariatric Surgery
Finally, there was a number of patients (5%) who underwent bariatric surgery (BS) in an attempt to lower body weight. Obesity is a known risk factor for multiple other spinal pathologies including spondylosis, spinal stenosis, and spondylolisthesis with Luike et al reporting BMI >25 increases risk of degenerative lumbar disease [42-46]. Two studies evaluating SIJ fusion in the context of BMI that both demonstrated obese and non-obese patient groups demonstrating similar improvements in terms of pain and function after SIJ fusion [21,47]. In exploring the correlation between improved back pain and bariatric surgery, some authors have proposed that the overall improvement in back pain following surgery may be attributed to alterations in metabolic efficiency as opposed to mechanical or musculoskeletal changes. Multiple studies [48,50], have demonstrated that substantial weight loss after bariatric surgery is associated with a significant decrease in VAS (p< 0.001) and ODI (p< 0.001). However, other studies argue that the improvement in back pain is more singularly dependent on reduced axial load (i.e., spinal biomechanics, reduction in local inflammatory processes from mechanical loading, off-loading of the intervertebral disc or facet joints) leading to symptom improvement in VAS and ODI scores.
Previous literature has characterized many micronutrient deficiencies that often occur in BS patients. Post BS patients are at increased risk of bone fractures and have increased markers of bone turnover as these changes in bone quality may be related to decreased overall BMD in obese patients that have undergone BS. As a result, there may be a greater risk of longer-term complications among patients with prior BS, such as pseudoarthrosis and worse patient-reported outcomes have yet to be investigated [51]. Although weight loss from BS has the potential to improve spine surgery outcomes, its detrimental effects on bone density due to nutritional deficits may pose a challenge [52,53]. As a result, it is unclear as to what effect, prior BS has on complications after spine surgery. Although reasonably convincing data have shown that patients with obesity have worse clinical outcomes and higher risks of postoperative complications it is not well established whether BS would mitigate these risks in patients undergoing spinal surgery.
LIMITATIONS
A limitation of our study is the heterogeneity of various non-operative treatments such as physical therapy given the significant variation in techniques used, exercises performed, and muscle groups targeted. Patient participation in therapy both clinical and in performing home-exercises as well as physical therapist experience in treating SIJ pain can also contribute to the heterogeneity and results of this management strategy. Additionally, injections are also subject to variation based on location of injection whether intra- or extra-articular, the formulation of anesthetic and/or corticosteroid used as well as volume. Other limitations that might affect generalizability include study participants being from a single institution and selection bias given only participants with complete records were included.
CONCLUSION
The findings suggest that anthropometrics may not influence improvement in patient-reported outcomes and obesity should not be considered as a contraindication to surgical management for patients where the main goal is to obtain adequate functional improvement.
STATEMENTS AND DECLARATIONS
IM, BR, MB and KO, have nothing to disclose. DWP declares consulting fees from Globus Medical and Alexion; institutional grant/research support from Medtronic and Mizuho OSI; consulting fees, royalties, and honoraria from SI Bone; and royalties from Springer.
ACKNOWLEDGEMENTS
We would like to thank Ayman Emara MD for his proofreading and editing services.
REFERENCES
- Sembrano JN, Polly DW. How often is low back pain not coming from the back? Spine. 2009; 34: E27-32.
- Bernard T. The role of the sacroiliac joints in low back pain: basic aspects of pathophysiology, and management. In: Movement, stability, and low back pain: the essential role of the pelvis. New York: Churchill Livingstone; 1997; 73-88.
- Liliang PC, Lu K, Liang CL, Tsai YD, Wang KW, Chen HJ. Sacroiliac joint pain after lumbar and lumbosacral fusion: findings using dual sacroiliac joint blocks. Pain Med. 2011; 12: 565-570.
- DePalma MJ, Ketchum JM, Saullo TR. Etiology of Chronic Low Back Pain in Patients Having Undergone Lumbar Fusion. Pain Med. 2011; 12: 732-739.
- Polly DW, Swofford J, Whang PG, Frank CJ, Glaser JA, Limoni RP, et al. Two-Year Outcomes from a Randomized Controlled Trial of Minimally Invasive Sacroiliac Joint Fusion vs. Non-Surgical Management for Sacroiliac Joint Dysfunction. Int J Spine Surg. 2016; 10: 28.
- Dengler J, Duhon B, Whang P, Frank C, Glaser J, Sturesson B, et al. Predictors of Outcome in Conservative and Minimally Invasive Surgical Management of Pain Originating from the Sacroiliac Joint: A Pooled Analysis. Spine (Phila Pa 1976). 2017; 42: 1664-1673.
- Finkelstein EA, Trogdon JG, Cohen JW, Dietz W. Annual medical spending attributable to obesity: Payer-and service-specific estimates. Health Aff (Millwood). 2009; 28: w822-831.
- Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011-2012. JAMA. 2014; 311: 806-814.
- Saini S, Bono O, Li L, MacAskill M, Chilton M, Ross G, et al. Investigating a Potential Limit to Access to Care: Preoperative Cutoff Values for Body Mass Index for Shoulder Arthroplasty. J Am Acad Orthop Surg. 2022; 30: e67-e73.
- Vaidya R, Carp J, Bartol S, Ouellette N, Lee S, Sethi A. Lumbar spine fusion in obese and morbidly obese patients. Spine (Phila Pa 1976). 2009; 34: 495-500.
- Soroceanu A, Burton DC, Diebo BG, Smith JS, Hostin R, Shaffrey CI, et al. Impact of obesity on complications, infection, and patient- reported outcomes in adult spinal deformity surgery. J Neurosurg Spine. 2015; 23: 656-664.
- Alsoof D, Johnson K, McDonald CL, Daniels AH, Cohen EM. Body Mass Index and Risk of Complications after Posterior Lumbar Spine Fusion: A Matched Cohort Analysis Investigating Underweight and Obese Patients. J Am Acad Orthop Surg. 2023; 31: e394-e402.
- Cohen SP. Sacroiliac joint pain: a comprehensive review of anatomy, diagnosis, and treatment. Anesth Analg. 2005; 101: 1440-1453.
- Gartenberg A, Nessim A, Cho W. Sacroiliac joint dysfunction: pathophysiology, diagnosis, and treatment. Eur Spine J. 2021; 30: 2936-2943.
- Boukovalas S, Boson AL, Padilla PL, Sljivich M, Tran JP, Spratt H, et al. Insurance Denials in Reduction Mammaplasty: How Can We Serve Our Patients Better? Plast Reconstr Surg. 2020; 146: 127e-136e.
- Buchanan P, Vodapally S, Lee DW, Hagedorn JM, Bovinet C, Strand N, et al. Successful Diagnosis of Sacroiliac Joint Dysfunction. J Pain Res. 2021; 14: 3135-3143.
- Copay AG, Cher DJ. Is the Oswestry Disability Index a valid measure of response to sacroiliac joint treatment? Qual Life Res. 2016; 25: 283-292.
- Fairbank JC, Pynsent PB. The Oswestry Disability Index. Spine. 2000; 25: 2940-2952.
- Copay AG, Subach BR, Glassman SD, Polly DW Jr, Schuler TC. Understanding the minimum clinically important difference: a review of concepts and methods. Spine J. 2007; 7: 541-546.
- Copay AG, Glassman SD, Subach BR, Berven S, Schuler TC, Carreon LY, et al. Minimum clinically important difference in lumbar spine surgery patients: a choice of methods using the Oswestry Disability Index, Medical Outcomes Study questionnaire Short Form 36, and pain scales. Spine J. 2008; 8: 968-974.
- Beckmann M, Odland K, Polly DW Jr. A retrospective cohort review of BMI on SI joint fusion outcomes: examining the evidence to improve insurance guidelines. Eur Spine J. 2024.
- Luukkainen RK, Wennerstrand PV, Kautiainen HH, Sanila MT, Asikainen EL. Efficacy of periarticular corticosteroid treatment of the sacroiliac joint in non-spondylarthropathic patients with chronic low back pain in the region of the sacroiliac joint. Clin Exp Rheumatol. 2002; 20: 52-54.
- Luukkainen R, Nissila? M, Asikainen E, Sanila M, Lehtinen K, Alanaatu A, et al. Periarticular corticosteroid treatment of the sacroiliac joint in patients with seronegative spondylarthropathy. Clin Exp Rheumatol. 1999; 17: 88-90.
- Maugars Y, Mathis C, Berthelot JM, Charlier C, Prost A. Assessment of the efficacy of sacroiliac corticosteroid injections in spondylarthropathies: a double-blind study. Br J Rheumatol. 1996; 35: 767-770.
- Karabacakoglu A, Karaköse S, Ozerbil OM, Odev K. Fluoroscopy- guided intra-articular corticosteroid injection into the sacroiliac joints in patients with ankylosing spondylitis. Acta Radiol. 2002; 43: 425-427.
- Fritz J, König CW, Günaydin I, Clasen S, Kastler B, Kotter I, et al. Magnetic resonance imaging – guided corticosteroid-infiltration of the sacroiliac joints: pain therapy of sacroiliitis in patients with ankylosing spondylitis. Rofo. 2005; 177: 555-563.
- Schneider BJ, Huynh L, Levin J, Rinkaekan P, Kordi R, Kennedy DJ. Does Immediate Pain Relief After an Injection into the Sacroiliac Joint with Anesthetic and Corticosteroid Predict Subsequent Pain Relief? Pain Medicine. 2018; 19: 244-251.
- Slipman WC, Lipetz SJ, Plastaras TC, Jackson HB, Vresilovic EJ, Lenrow DA, et al. Fluoroscopically Guided Therapeutic Sacroiliac Joint Injections for Sacroiliac Joint Syndrome. Am J Phys Med Rehabil. 2001; 80: 425-432.
- Polly DW, Swofford J, Whang PG, Frank CJ, Glaser JA, Limoni RP, et al. Two-Year Outcomes from a Randomized Controlled Trial of Minimally Invasive Sacroiliac Joint Fusion vs. Non-Surgical Management for Sacroiliac Joint Dysfunction. Int J Spine Surg. 2016; 10: 28.
- Schmidt GL, Bhandutia AK, Altman DT. Management of Sacroiliac Joint Pain. J Am Acad Orthop Surg. 2018; 26: 610-616.
- Murakami E, Tanaka Y, Aizawa T, Ishizuka M, Kokubun S. Effect of periarticular and intraarticular lidocaine injections for sacroiliac joint pain: prospective comparative study. J Orthop Sci. 2007; 12: 274-280.
- Murakami E, Kurosawa D, Aizawa T. Treatment strategy for sacroiliac joint-related pain at or around the posterior superior iliac spine. Clin Neurol Neurosurg. 2018; 165: 43-46.
- Nilsson-Wikmar L, Holm K, Oijerstedt R, Harms Ringhdal K. Effect of three different physical therapy treatments on pain and activity in pregnant women with pelvic girdle pain: a randomized clinical trial with 3, 6, and 12-months follow-up postpartum. Spine. 2005; 30:850-856.
- Delitto A, Cibulka MT, Erhard RE, Bowling RW, Tenhula JA. Evidence for use of an extension-mobilization category in acute low back syndrome: a prescriptive validation pilot study. Phys Ther. 1993; 73: 216-222.
- Cohen PS, Hurley WR, Buckenmaier CC, Curihara C, Morlando B, Dragovich A. Randomized Placebo-controlled Study Evaluating Lateral Branch Radiofrequency Denervation for Sacroiliac Joint Pain. Anesthesiology. 2008; 109: 279-288.
- Patel N, Gross A, Brown L, Gekht G. A Randomized, Placebo-Controlled Study to Assess the Efficacy of Lateral Branch Neurotomy for Chronic Sacroiliac Joint Pain. Pain Med. 2012; 13: 383-398.
- Slipman CW, Jackson HB, Lipetz JS, Chan KT, Lenrow D, Vresilovic EJ. Sacroiliac joint pain referral zones. Arch Phys Med Rehabil. 2000; 81: 334-338.
- Patel N. Twelve-Month Follow-Up of a Randomized Trial Assessing Cooled Radiofrequency Denervation as a Treatment for Sacroiliac Region Pain. Pain Pract. 2016; 16: 154-167.
- Ferrante FM, King LF, Roche EA. Radiofrequency sacroiliac joint denervation for sacroiliac syndrome. Reg Anesth Pain Med. 2001; 26: 137-142.
- Aydin SM, Gharibo CG, Mehnert M, Stitik TP. The role of radiofrequency ablation for sacroiliac joint pain: a meta-analysis. PM R. 2010; 2: 842- 851.
- Polly D, Mundis G, Eastlack R, Levegue JC, Elder BD, Martin C, et al. Randomized Trial of Augmented Pelvic Fixation in Patients Undergoing Thoracolumbar Fusion for Adult Spine Deformity: Initial Results from a Multicenter Randomized Trial. World Neurosurg. 2024; 2: S1878-8750
- Liuke M, Solovieva S, Lamminen A, Luoma K, Arjas PL, Luukkonen R, et al. Disc degeneration of the lumbar spine in relation to overweight. Int J Obes (Lond). 2005; 29: 903-908.
- Lingutla KK, Pollock R, Benomran E, Purshothaman B, Kasis A, Bhatia CK, et al. Outcome of lumbar spinal fusion surgery in obese patients: a systematic review and meta-analysis. Bone Joint J. 2015; 97: 1395- 1404.
- Goyal A, Elminawy M, Kerezoudis P, Lu VM, Yolcu Y, Alvi MA, et al. Impact of obesity on outcomes following lumbar spine surgery: A systematic review and meta-analysis. Clin Neurol Neurosurg. 2019; 177: 27-36.
- Rosen DS, Ferguson SD, Ogden AT, Huo D, Fessler RC. Obesity and self-reported outcome after minimally invasive lumbar spinal fusion surgery. Neurosurg. 2008; 63: 956-960.
- Lau D, Khan A, Terman SW, Yee T, Marca FL, Park P. Comparison of perioperative outcomes following open versus minimally invasive transforaminal lumbar interbody fusion in obese patients. Neurosurg Focus. 2013; 35: E10.
- Odland K, Cher D, Polly DW. Effects of BMI on SI joint fusion outcomes: examining the evidence to improve insurance guidelines. Spine J. 2024; 24: 783-790.
- Stefanova I, Currie AC, Newton RC, Albon L, Slater G, Hawkins W, et al. A Meta-analysis of the Impact of Bariatric Surgery on Back Pain. Obes Surg. 2020; 30: 3201-3207.
- Lidar Z, Salame K. Minimally invasive posterior cervical discectomy for cervical radiculopathy: technique and clinical results. J Spinal Disord Tech. 2011; 24: 521-524.
- Khoueir P, Black MH, Crookes PF, Kaufman HS, Katkhouda N, Wang MY. Prospective assessment of axial back pain symptoms before and after bariatric weight reduction surgery. Spine J. 2009; 9: 454-463
- Gupta S, Tao X, Matur AV, Wu A, Chilkapati SS, Palmisciano P, et al. Bariatric Surgery Before Spine Surgery is Associated with Fewer Postsurgical Complications: A Systematic Review and Meta-Analysis. Spine (Phila Pa 1976). 2023; 48: 944-949.
- Gagnon C, Schafer AL. Bone health after bariatric surgery. JBMR Plus. 2018; 2: 121-133.
- Lupoli R, Lembo E, Saldalamacchia G, Avola CK, Angrisani L, Capaldo B. Bariatric surgery and long-term nutritional issues. World J Diabetes. 2017; 8: 464.