5-Methyltetrahydrofolate Content of Cereal-Based Processed Foods
- 1. Formerly Graduate Research Assistant South Dakota State University, Cereal Science and Dough Technology Nestlé R&D Center, USA
- 2. Medallion Laboratories Division, General Mills Inc., USA
- 3. Health and Nutritional Sciences Department, South Dakota State University, USA
Abstract
Folates are important for human nutrition and health. While certain foods have a significant amount of naturally occurring folate (predominantly 5-Methyltetrahydrofolate, 5-MeTHF), other foods may be fortified with synthetic folic acid. 5-MeTHF and folic acid have comparable physiological activity, however, intake of 5-MeTHF may have advantages over intake of folic acid in terms of reducing the potential for masking of vitamin B12 deficiency and interaction with drugs that inhibit dihydrofolate reductase. Hence, an optimized method to measure 5-MeTHF in foods was developed and used to measure it in selected processed and ready-to-eat (RTE) foods. The optimized method of extraction, purification and chromatographic separation yielded good recovery of 5-MeTHF (107.1%) in samples. A tripleenzyme digestion procedure accomplished de-proteinization, starch-degradation, and deconjugation of the folate analytes. A Solid Phase Extraction (SPE) device was used to purify sample extracts in a strong anion exchange mode. An isocratic High Performance Liquid Chromatography (HPLC) procedure employing fluorimetric detection was effective in separating and quantifying 5-MeTHF within a 30-minute run-time. The chromatograms of food samples showed well-resolved 5-MeTHF peaks after 19 to 21 minutes retention time. Average 5-MeTHF content of selected food samples ranged from 3 to 32 µg/100g. 5-MeTHF content of cheeses ranged between 5 to 9 µg/100g, while it was 32 µg/100g for milk powder. Frozen vegetables contained 20-31µg/100g of 5-MeTHF. RTE cereals or cereal products contained 4 to 11 µg/100g of 5-MeTHF; with cereal containing the bran having higher amount than their bran-free counterparts. The optimized method can be used to quantify naturally occurring 5-MeTHF in different food matrices; and allow consumers to differentiate between the naturally occurring folate forms and added folic acid in food.
Keywords
• Folate
• 5-Methyltetrahydrofolate
• Natural folate
• Processed foods
• RTE cereals
• HPLC
• Solid phase extraction
• Fluorimetric analysis
Citation
Amornkul Y, DeVries JW, Krishnan PG (2013) 5-Methyltetrahydrofolate Content of Cereal-Based Processed Foods. J Hum Nutr Food Sci 1(2): 1010
INTRODUCTION
Improving periconceptional folate status reduces the risk of neonatal neural tube defects. Therefore, increased folate intake is recommended before and during the early stages of pregnancy through folic acid supplements or fortified foods. Folates also have a role in prevention of other diseases such as Cardiovascular, Cancers, Alzheimer [1]. Folates exist in a variety of forms related to the parent compound folic acid (pteroylglutamic acid, PGA) and each possesses different biological activity. Folic acid is produced synthetically and is only found in fortified foods and supplements [2]. The most common forms of naturally occurring folates found in foods are 5-methyltetrahydrofolic acid (5-MeTHF), tetrahydrofolic acid (THF) and 5-formyl tetrahydrofolic acid (5CHOTHF). Of these 5-MeTHF is the predominant folate vitamer found naturally in foods [3].
Folic acid, found in fortified foods, lacks coenzyme activity and must be reduced to the metabolically active tetrahydrofolate form with in the cell [1].
5-MeTHF, the predominant natural folate found in food and blood, is the folate form that is transported into peripheral tissues to be used for cellular metabolism. Studies have found that 5-MeTHF and folic acid have comparable physiological activity, bioavailability, and absorption at equimolar doses [1]. However, intake of 5-MeTHF may have advantages over intake of folic acid in terms of reducing the potential for masking of vitamin B12 deficiency and interaction with drugs that inhibit dihydrofolate reductase [1]. Consequently, there is a need to provide a database on 5-MeTHF form of folate in food products, in addition to more frequently reported total folate content.
The existing data for folate content in foods is based on the microbiological assay, which provides an estimate of total folate content and not of the individual folate compounds. High Performance Liquid Chromatography (HPLC) methods allow for determination of individual folate (including 5-MeTHF) in a variety of difficult sample matrices [4-9,2,3,10]. HPLC data are, however, not well represented in the U.S. food composition databases. This is due to the elaborate nature of the sample extraction techniques, complicated purification and enrichment schemes, and the need for validated techniques optimized for varied sample matrices. The use of solid phase extraction (SPE) columns for selective purification have gained popularity as they are relatively inexpensive, show promise for trace enrichment and when used carefully, yield adequate sample purification and quantitative recovery of target compounds [8,11].
The objective of this study was to develop an optimized HPLC method to quantify 5-MeTHF in food samples and report values of 5-MeTHF in selected food samples.
MATERIALS AND METHODS
Samples
A variety of dairy products (American and Swiss cheeses, Carnation? non-fat dry milk), Frozen vegetables (Green Giant? mixed vegetables, green pea, broccoli), Ready-to Eat cereals (Cheerios?, Kellogg?’ s Product 19, Kellogg?’ s Special K), and dry pasta (enriched macaroni, enriched spaghetti) were purchased from a local grocery store. RTE cereal samples and flours representing a variety of cereal grains and used in a previous inter-laboratory collaborative study were also assayed [12]. Dried samples were ground with a mortar and pestle. Cheese and frozen vegetables were ground using a food processor. All food samples were stored at –18 °C in airtight containers until analysis.
Preparation of Standard
5-MeTHF standard was obtained from Dr. Schirck’s Laboratories (Jona, Switzerland). A stock standard solution of 5- MeTHF was prepared by dissolving five milligrams of 5- MeTHF in 100 ml of 0.1 M sodium acetate containing 10% (w/v) sodium chloride and 1% (w/v) ascorbic acid in a volumetric flask. Working standard solutions of 1.25, 2.5, 5.0, 7.5, 10.0, 12.5, 15.0, 17.5, 22.5, and 25.0 ng/mL were obtained through serial dilution of stock solution. All standard solutions were stored at -18 °C to minimize degradation.
Sample extraction and purification
An extraction/purification method for 5-MeTHF in food samples was optimized based on published methods [9,13,8,11]. All extraction and purifications steps were conducted under gold fluorescent light to minimize destruction of folate. Two grams of the ground food sample was mixed with 20 ml of Hepes/Ches buffer, pH 7.85 (50 mM Hepes and 50 mM Ches), containing 2% (w/v) sodium ascorbate and 10 mM 2-mercaptoethanol in a 50 ml centrifuge tube. After mixing with a vortex mixer, all the tubes were placed in a boiling water bath for 10 minutes, cooled rapidly in an ice-bath, and then homogenized using a tissue homogenizer (Biospec Products, Bartlesville, OK) for 1 minute. The contents of the tubes were then subjected to a trienzyme treatment. This included inoculation of each tube with 0.5 mL of rat plasma folate conjugase and 1 mL of α-amylase and incubation at 37°C for 4 hours, followed by inoculation with 2 mL of protease and incubation at 37°C for 1 hour. At the end of enzyme treatments, the tubes were heated in a boiling water bath for 5 minutes, cooled in an ice-bath, and centrifuged at 5000 x g for 10 minutes at 5 °C. The pellet was re-dissolved in 5 ml of extraction buffer (HEPES/CHES buffer) and re-centrifuged at 5,000 x g for 10 minutes at 5°C. The supernatants were then combined together, mixed well, and stored at 4°C until further purification.
A 3-mL strong ion exchange column – SPE (Bakerbond SPE, catalogue No.7091-3, J.T. Baker, NJ) was used to purify and concentrate the sample extracts using a Baker vacuum manifold. Three milliliters each of hexane, methanol, and de-ionized water were sequentially used to activate each cartridge. The cartridge was then equilibrated with 10 ml of 0.01 M phosphate buffer containing 0.1 % 2-mercaptoethanol (pH 7.0 conditioning buffer). A 3-mL aliquot of sample extract was diluted with 6-mL de-ionized water, and 15 µL of 2-mercaptoethanol before application on to the SPE column. The mixture was slowly passed at 33-35 drops per minute flow rate through the SPE column using the vacuum manifold set for 10 KPa. The column was then washed with 3 ml of conditioning buffer, and the folate compounds were eluted with 3 ml of 0.1 M sodium acetate containing 10% (w/v) sodium chloride and 1% (w/v) ascorbic acid. The final eluate was mixed vigorously using a vortex mixer and refrigerated at 4°C until HPLC analysis.
HPLC Separation and Analysis
An isocratic HPLC separation similar to that reported by Day and Gregory (1981) was employed. Separation of 5-MeTHF analysis was performed on a system consisting of a Waters 515 HPLC pump (Waters Corporation, Milford. MA), Rheodyne injector (Model 7125, Upchurch Scientific, Inc., Oak Harbor, WA), equipped with 100 µl injection loop, a Jasco Model 82 1-Intelligent Spectrofluorometric detector (JASCO, Japan, Ex=290nm & Em=356nm, Gain= 100) and a Model 3390A Integrator (Hewlett Packard Co., Avondale, PA). The analytical column used was a Genesis C18 column with 250 x 4.6mm i.d., and 4 µm particle size (Jones Chromatography, Lakewood, CO) protected by a 1-cm × 4-mm guard column (Genesis C18 , 4-µm particle size, Jones Chromatography, Lakewood, CO). The column was operated at ambient temperature, using an isocratic mobile phase of 6% acetonitrile and 94% of 33 mM phosphoric acid (pH 2.3) and a flow rate 1.0 ml/min (this condition was selected for the best possible separation at a reasonably short retention time which is18-21minutes). All samples were filtered through a 0.45 µm filter (Millipore Corporation, Bedford, MA) prior to HPLC analysis. Quantification was based on an external standard method in which the peak area was plotted against the concentration of the standard and using the equation given below. Calibration plots using least-squares regression analysis were also prepared for 5- MeTHF standard in the concentration range of 1.25, 2.5, 5.0, 7.5, 10.0, 12.5, 15.0, 17.5, 22.5, and 25.0 ng/ml. Working standard solutions were prepared fresh by diluting stock solutions on the same day that samples were prepared.
RESULTS AND DISCUSSION
Optimized Method
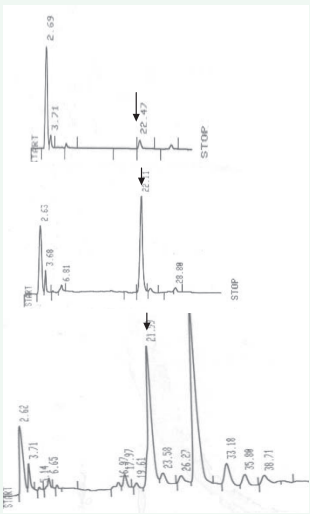
Extraction and Purification: Additional peaks other than the clearly resolved 5-MeTHF were noted in the chromatogram. These peaks were originating in the amylase and protease incubation steps but not if only the polyglutamase digestion is carried out. This is graphically demonstrated in figure 3 where an extraneous and large post-5-MeTHF eluting peak is noted only with the additional amylase and protease treatments. Figure 3 also shows that protease and rat plasma polyglutamase enzyme incubation are necessary for more complete recovery of 5-MeTHF as judged by the chromatogram of milk powder sample. This is consistent with earlier work by others [14-17,9]. Food samples, however, are more recalcitrant in yielding their true endogenous levels of folate and will benefit from this step.
Figure 3: Role of folate conjugase enzyme from rat plasma in 5-MeTHF measurement. Chromatograms of 5-MeTHF in milk powder.
• First chromatogram-No use of enzyme
• Second chromatogram-Use of rat plasma folate conjugase treatment alone,
• Third chromatogram-Use of trienzyme treatment (alpha amylase, protease, and rat plasma folate conjugase enzyme)
SPE sample purification step was also observed to be a critical step in the analysis. Sample extract dilution with water prior to SPE loading, 1:2 (v/v) as recommended by Vahteristo et al (1996) proved to be an effective step in insuring low salt concentration and adequate retention of the target compound on to the SPE cartridge. The use of the SPE clean-up was necessary for most samples although extraneous peaks which did not interfere with the analyte of interest were still noted in the chromatograms. SPE using commercial strong anion exchange columns proved to give complete recovery of pure standards. We have determined that up to 5 microgram of 5-MeTHF can be loaded onto the SPE column and recovered without losses [18]. This is a considerably larger binding capacity when contrasted to the reported upper limit values for folate-binding capacity of affinity columns Konings (1999) who recommended a folate load of no more than 2.04 µg for FBP affinity columns. Commercial SPE cartridges continue to prove to be effective tools in the cleanup of samples prior to chromatography for endogenous 5-MeTHF determination [18,19].
HPLC: 5-MeTHF peaks were adequately resolved for peak quantification. Retention times for 5-MeTHF on the Genesis column varied between 19 and 21 minutes, and this drift did not affect peak identification within a given run. A detection limit of 0.625ng/mL was noted for 5-MeTHF in a pure standard, with the use of a 100-µL injection loop volume. An isocratic HPLC system proved effective. The occurrence of late eluting peaks after 5-MeTHF elution (30 minutes) in most samples may warrant a gradient separation for speedier clean up and re-equilibration of the column prior to the analysis of the next sample.
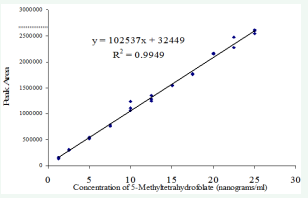
Pure standards made up in the working range of estimated sample folate concentration and taken through the entire analytical protocol showed good linearity as judged by high correlation coefficients (R2 = 0.9949, figure 1) between concentration and fluorescence detector response.
Figure 1: Standard curve of 5-methyltetrahydrofolate . Fluorescence response to 100 microliter injections of standards taken through extraction and clean up procedure.
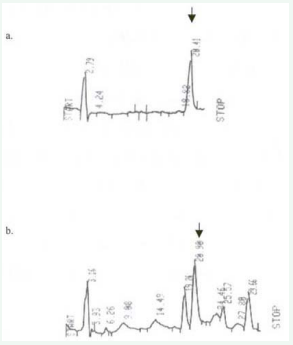
Pure standards also showed good recovery, almost 100 %, when they were subjected to enzymatic digestion, extraction, and SPE purification (figure 2) to simulate sample treatment.
Figure 2: Chromatograms showing: (a) Pure 5- MeTHF standard (27.8 ng/ml, Peak Area 3,585,200) . (b) The same standard taken through the entire folate analytical procedure as food sample had Peak Area 3,687,400.
Spiking experiments using food samples showed that 5-MeTHF added to samples prior to extraction yielded recovery of 98.7, 107.9,103.7, 101.1, 131.1, and 100.1% for milk powder, frozen mix vegetables, Cheerios®, Kellogg’s® Product 19, Kellogg’s® Special K, and enriched macaroni, respectively (table 1).
Table 1: Percentage recovery of spiked 5-Methyltetrahyrdrofolate in some food samples.
| Samples | % Recovery | Standard Deviation |
| Milk Powder | 98.7 | 10.6 |
| Frozen Mixed Vegetables | 107.9 | 7.50 |
| Cheerios® | 103.7 | 23.9 |
| Kellogg’s® Product 19 | 101.1 | 4.00 |
| Kellogg’s® Special K | 131.1 | 7.8 |
| Macaroni | 100.1 | 8.90 |
| Average Recovery % | 107.1 | |
The average recovery of added 5-MeTHF was 107.1%.
MeTHF content of selected foods
The 5-MeTHF concentration in selected cheeses, frozen vegetables, milk powder, cereals, and flours are presented in table 2.
Table 2: 5-Methyltetrahydrofolate content (µg/100g) of Selected Food Products (average of 3 samples)
| Food products | 5-MeTHF content | Standard Deviation |
| Swiss Cheese (fresh) * | 5.63 | 0.29 |
| American Cheese (fresh) * | 9.53 | 0.67 |
| Milk Powder (Carnation) * | 31.90 | 2.60 |
| Frozen mixed vegetables* | 31.30 | 4.00 |
| Frozen Broccoli* | 17.96 | 3.18 |
| Frozen Green peas* | 20.56 | 2.66 |
| Enriched macaroni* | 5.00 | 0.70 |
| Cookies (High fat) | 15.30 | 1.30 |
| Cookies (Low fat) | 5.14 | 1.51 |
| Cookies (High fiber) | 3.03 | 0.70 |
| Unfortified white flour | 16.78 | 1.91 |
| Enriched white flour | 11.68 | 1.06 |
| Whole wheat flour | 5.22 | 1.56 |
| Enriched white bread | 14.05 | 1.52 |
| Spaghetti (uncooked) | 10.17 | 4.36 |
| Enriched pasta | 8.45 | 1.33 |
| Baking mix | 19.68 | 6.39 |
| Corn tortilla | 6.39 | 2.43 |
| Corn Meal | 15.32 | 3.61 |
| RTE cereal-wheat bran | 10.89 | 2.19 |
| RTE Cereal-wheat | 4.10 | 2.31 |
| RTE Cereal-oat | 7.61 | 2.35 |
| RTE Cereal-rice | 4.18 | 0.87 |
| Cheerios®* | 8.70 | 0.60 |
| Kellogg’s® Product 19* | 9.20 | 1.70 |
| Kellogg’s® Special K* | 5.70 | 1.70 |
Frozen green pea, mixed vegetables, and milk powder showed higher levels of 5-MeTHF (20.6, 31.3, 31.9 µg/100g) when compared to other samples. Milk powder showed a folate content of 31.9 µg/100g. This value is comparable to 5-MeTHF of 15.7 to 29.8 µg/100g reported by Konings (1999) [2] for spray dried cow’s milk powder. 5-MeTHF content of cheeses ranged between 5 to 9 µg/100g. RTE breakfast cereals had concentrations of 5-MeTHF of 4.1 to 10.9µg /100 grams. Po´oPrieto et al (2006) [20] reported content of 5-MeTHF in RTE cereals such as Cheerios® and Kellogg’s® Rice Krispies (RTE cereal rice) were 12.8 and 2.2 µg/100g. These two numbers were close to our results. Other grain-based foods analyzed in this study had 4.2 to 16.8 µg/100g 5-MeTHF. Limited published information exists on 5-MeTHF content in comparable products to allow for direct comparisons. Pfeiffer and coworkers (1997) [9] reported 5-MeTHF concentrations of 8.5, 4.9 and 3.4 µg/100g for white bread, white rice, and spaghetti, respectively, using affinity chromatography and a trienzymic HPLC method. Other values of 5-MeTHF content reported for raw spaghetti, corn meal, and corn tortilla (cooked taco shell) were 9.4, 28 and 11.5 µg/100g respectively [20]. Holasova et al [21] reported 5-MeTHF concentrations of 56 µg/100g in raw broccoli.
CONCLUSION
This study presents an optimized analytical protocol for the measurement of 5-MeTHF in processed cereal based foods and selected dairy and vegetable products. 5- MeTHF was extracted from the sample after trienzymic digestion/incubation, purified using strong anion exchange chromatography prior to quantification by isocratic HPLC. The optimized method can help chemists quantify naturally occurring 5-MeTHF in different food matrices and allow consumers to differentiate between the naturally occurring folate forms and added folic acid in food.
ACKNOWLEDGEMENT
This study was supported by the SD Agricultural Experiment Station, USDA National Research Initiative Competitive Grants Program and the Ethel Austin Martin Nutrition Program Mini Grant, South Dakota State University.