A Simple and Efficient Method For In Vitro Growth Detection of Intestinal Bacteria Treated with Drugs
- 1. College of Forestry and Biotechnology, Zhejiang Agriculture and Forestry University, China
- 2. Zhejiang Academy of Forestry, China
- 3. Key Laboratory of National Forestry and Grassland Administration on Forest Food Resources Utilization and Quality Control, China
- #. Author contributed equally
Abstract
Objectives: To develop a cost-effective anaerobic culture method for intestinal bacteria using multi-layer liquid seal and chemical adsorption under aerobic conditions.
Results: The proposed method successfully cultivated anaerobic bacteria, including Clostridium butyricum, Bifidobacterium longum, and Enterococcus faecalis, demonstrating its efficacy. Fructose and procyanidin were tested, showing positive effects on bacterial growth. Multi-day transfer cultures of mixed intestinal bacteria confirmed the method’s repeatability and reliability, enabling efficient in vitro growth detection of numerous intestinal bacteria using standard anaerobic media.
Conclusions: A novel, simple anaerobic culture method under aerobic conditions enables cost-effective and reliable growth detection of intestinal bacteria for drug effect comparisons
KEYWORDS
- Anaerobic culture
- Bacteria growth detection
- Intestinal bacteria
CITATION
Liu XY, Liu XF, Peng HZ, Zhu TJ(2024) A Simple and Eficient Method For In Vitro Growth Detection of Intestinal Bacteria Treated with Drugs. J Hum Nutr Food Sci 12(3): 1191
INTRODUCTION
Human intestinal bacteria grow in a hypoxic environment, and the huge metabolic diversity of these anaerobic bacteria is very important for human health and industrial use [1]. Because these bacteria are sensitive to oxygen, it is difficult to operate, culture and detect in a conventional aerobic environment. The commercial anaerobic workstation is a reliable device for the treatment of anaerobic microorganisms. In the device, not only the routine operation of strain isolation and culture can be carried out, but also the growth detection instrument such as microplate reader can be placed inside, and the growth of anaerobic bacteria can be determined by microplate [2]. However, the purchase and maintenance costs of such devices are high, and the operation is also cumbersome. Therefore, simple anaerobic operation methods or devices are an area of concern, especially for laboratories with limited economic conditions. In the operation and culture of anaerobic bacteria, the most direct method is to seal microbial samples in airtight containers and use chemical reactions to adsorb oxygen, such as the anaerobic technology developed by Robert Hungate, which is based on the formation of inert gases such as nitrogen and carbon dioxide from anoxic gases in the air through a heated copper column (pre-reduced with H2) [3]. The subsequent use of this principle to establish the filling station, although it is convenient for the rapid preparation of anaerobic medium, without the need for bulky anaerobic tanks and other equipment, but for the researchers, the installation and maintenance of the filling station is also a big challenge [4,5]. Of course, designing a lower-cost anaerobic operation box is also a good choice, which can realize some functions of the anaerobic workstation [6,7]. In general, these anaerobic cultivation and devices basically need to be carried out in anaerobic devices. Although the cost can be reduced by simplification, it is still much more complicated than conventional strain operation. More importantly, these simplified methods are difficult to detect the growth of large quantities of samples.
Microplate is a good device for the culture and detection of microorganisms in large quantities. Combined with microplate reader, a variety of chemical/biochemical reactions can be recorded in real time and quantitatively, including microbial growth. When used for anaerobic bacteria culture, an easy way to think of is to use paraffin oil as a liquid seal to avoid oxygen penetration into the wells. However, studies have shown that for 96-well microplates, even if the volume of paraffin oil per well is close to half of the pore volume, the covered anaerobic sample almost reaches air saturation after 2 hours [8]. In order to further create anaerobic conditions in microplates, a silicone paste is used to seal the lid and nitrogen is used to flush the microplate. The microplate was then cultured for 24 hours without shaking and the change of optical density was recorded in microplate reader [9]. In contrast, using a dedicated gas control module developed by some companies that can be used for microplate reader, the cost will be higher [10]. Of course, it is also possible to place the growth measurement device in the anaerobic device for direct measurement or wireless transmission, which is actually a simple alternative to the anaerobic workstation, and the production of device is not easy [11-13].
This method solved the problem of aerobic isolation under conventional experimental conditions by using multi-layer liquid seal, chemical adsorption and reasonable operation methods, which facilitates the culture and growth detection of anaerobic bacteria. Using this method, this paper studied the growth patterns of individual anaerobe and mixed intestinal microbiota under drugs, and verified their practicability and reliability.
MATERIALS AND METHODS
Samples, Strains, Drugs and Medium
Bifidobacterium longum, Clostridium butyricum and Enterobacter aerogenes were isolated from feces of healthy volunteers and identified by 16s rRNA sequencing. The stock solution of fructose (Sigma, USA) and procyanidin (Sigma, USA) was 5mM (about 40 times the working concentration). Fecal samples were taken from a healthy volunteer. YCFA was used as basic medium [14]. Pichia fermentans 15B1 was a patented strain of our laboratory (patent number: CN202110032614.8, preservation number in the China Microbial Culture Collection Center: CGMCC No.19317), Pichia fermentans 323 was purchased from the China Industrial Microbial Culture Collection Management Center Pichia fermentans P3238.
Strain Culture and Detection
Preparation of Oxygen-Removing Bag: Prepare a thick double-sealed plastic bag with a simple oxygen absorbent, which was mainly composed of iron powder, vermiculite, activated carbon and salt. It can be prepared in large quantities in advance and stored for months after sealing.
Preparation of gut microbiota: Add about 1g of feces to 10ml PBS solution containing deoxidizer.
Preparation of Test Microplate: Put the blank microplate in a oxygen-removing bag for more than 24 hours, add 150 μL YCFA liquid medium to each well of the microplate, and then add 20 μL liquid paraffin, vitamin E and silicone oil in turn. The culture plate in oxygen-removing bag was placed at 4? for later use. When the drugs were added, 20 μL of fresh 1 % cysteine solution was added together. After adding the drugs, cover the lid, put it back into the anaerobic bag, store at 4? overnight. Totally 8-12 replicates were set for each treatment.
Inoculation of Bacteria: Take out the bacteria in frozen tube from -80? refrigerator, thaw them at room temperature, add 4ml YCFA liquid medium and 500 μL 1 % cysteine solutionin Penicillin bottle, injected the intestinal bacteria into Penicillin bottle, seal the bottle with rubber plug and place it at 37? for overnight culture. Shake the bottle well before use, detect cell density with Ultrospec10 Cell Density Meter (GE, USA) to ensure OD600 is greater than 1.0, inoculate 15 μL culture into prepared microplates under ice bath, paste an optical film on the front of the microplate to reduce the air flow above the well, incubate the plate in the anaerobic bag at 37? for 30 min.
Detection and Collection of Samples: Microplates for microbial cultivation can be incubated at 37? in a microplate reader (Varioskan, USA), facilitating automatic detection of absorbance every 1-2 hours. The mixed intestinal microbiota was taken out from the anaerobic bag every 24 hours and then 15 μL bacterial suspension was transferred to new microplates under ice bath. The remaining bacterial in original microplates was frozen at-80 degrees for DNA extraction. The above transfer was repeated once at 48h, and finally intestinal bacteria samples at 24h, 48h and 72h were obtained for microbiota diversity analysis.
DNA Isolation and Sequencing
DNA Isolation: Centrifuge at 12000g for 1min to collect precipitation, wash with 150ul sterile water, repeat 2-3 times. Add 600 μL CTAB extract and 0.1mm zirconia grinding beads, grind them in MM400 grinder at 25HZ for 5min, incubate them at 65? for 15min, centrifuge at 12000g for 1min. Take 400 μL supernatant to the silicon adsorption column, add 200 μL ethanol to the column, centrifuge at 12000g for 1 min, add 500 μL 70 % ethanol, centrifuge at 12000g for 1min, add 50 μL TE (pH8.0), centrifuge at 12000 g for 1min, collect DNA and store at -80? for sequencing. DNA were amplified used the specific primer (16S rRNA V4-V5: 515F-907R) with the barcode. All PCR reactions were carried out in 30 μL reactions with 15 μL of Phusion®High-Fidelity PCR Master Mix (NEB, USA), 0.2μM of forward and reverse primers, and about 10 ng template DNA. Thermal cycling consisted of initial denaturation at 98? for 1 min, followed by 30 cycles of denaturation at 98? for 10 s, annealing at 50? for 30 s, and elongation at 72? for 60 s. Finally 72? for 5 min. Sequencing libraries were generated using NEB Next®Ultra™DNA Library Prep Kit for Illumina (NEB, USA) following manufacturer’s recommendations and index codes were added. The library quality was assessed on the Fluorometer (Thermo Scientific) and Agilent Bioanalyzer 2100 system. At last, the library was sequenced on an Illumina MiSeq platform and 250bp/300bp paired-end reads were generated.
Data Analysis: Paired-end reads from the original DNA fragments were merged using FLASH. Paired-end reads was assigned to each sample according to the unique barcodes. OUT cluster and species annotation Sequences analysis were performed by UPARSE software package using the UPARSE-OTU and UPARSE-OTUref algorithms. In-house Perl scripts were used to analyze alpha (within samples) and beta (among samples) diversity. Sequences with ≥ 97% similarity were assigned to the same OTUs. Data were further analyzed and graphs were drawn by excel and visio of office 2010 (Microsoft, USA).
RESULTS
Culture Testing of Individual Gut Bacteria
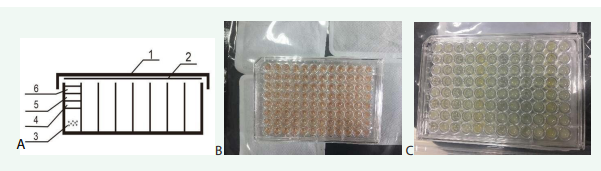
The design of the culture device is shown in Figure 1A.
Figure 1; A. Design of microplate device for anaerobic culture, 1 microplate cover, 2 optical film, 3 liquid medium, 4 silicone oil, 5Ve, 6 liquid paraffin ; B. Color of medium before deoxygenation; C. Color after deoxygenation and growth of bacteria
There are multiple layers of oily liquid on the liquid medium. Since the density order is liquid paraffin < Ve < silicone oil < water, they are naturally layered from top to bottom when they are added. The medium was red before the addition of deoxidizers and test reagents [Figure 1B]. After the addition of deoxidizers and reagents, the red faded after the pretreatment of deoxidization in a sealed bag containing deoxidizers [Figure 1C].
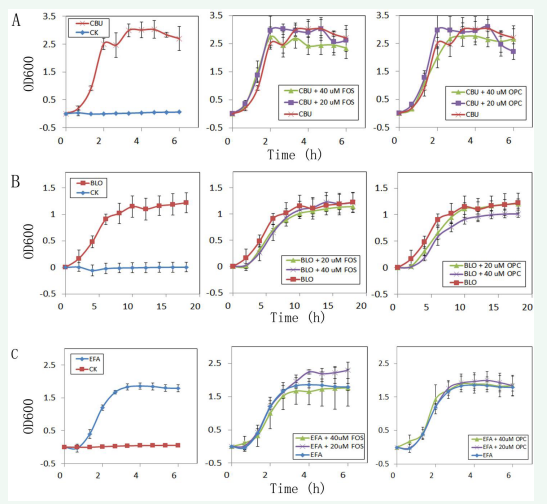
In order to verify the feasibility of intestinal bacteria culture, two strict anaerobic bacteria, Clostridium butyricum and Bifidobacterium longum, and a facultative anaerobic bacteria Enterococcus faecalis were selected for continuous culture and dynamic absorbance test. The results showed that the three bacteria had good growth in the microplate, and there was no significant change of absorbance in sterile control, indicating that this culture method can reflect the growth of the inoculated bacteria reliably [Figure 2].
Figure 2: A. Effects of fructose (FOS) and procyanidin (OPC) on the growth of Clostridium butyricum (CBU), CK as a sterile control ; B. Effects of FOS) and OPC on the growth of Bifidobacterium longum (BLO) , CK as a sterile control ; C. Effects of FOS and OPC on the growth of Enterococcus faecalis (EFA), CK as a sterile control.
The culture of Clostridium butyricum showed growth curves with four stages [Figure 2A]: lag phase, logarithmic phase, stable phase and decline phase. Clostridium butyricum completed its rapid proliferation process about 1h after the lag phase and entered the stable growth stage about 3h after the detection. The growth pattern of Bifidobacterium longum was different [Figure 2B]. Its rapid growth stage after lag phase was about 5h. After 10 hours of detection, it gradually entered the stable stage but was still slowly growing after 18 hours. The growth process of Enterococcus faecalis was similar to that of Clostridium butyricum [Figure 2C]. Its rapid growth time after lag phase was about 2h, and the stable phase was about 3h after detection.
The effects of FOS and OPC on the growth of three strains were also detected. The results showed that the presence of FOS could promote the rapid growth of C. butyricum in the logarithmic phase, but the high concentration of FOS could inhibit the growth of C. butyricum in the stationary phase. Similarly [Figure 2A]. OPC had little effect on the growth of C. butyricum. For B. longum [Figure 2B], in the rapid growth stage, both high and low concentrations of FOS or OPC have a certain inhibitory effect on its growth, but in the stationary phase, only high concentrations of OPC have a certain inhibitory effect on its growth. For E. faecalis [Figure 2C], in the rapid growth stage, both high and low concentrations of FOS or OPC had little effect on its growth, but in the stationary phase, low concentrations of FOS had a certain growth promoting effect.
Drug Test of Mixed Intestinal Bacteria
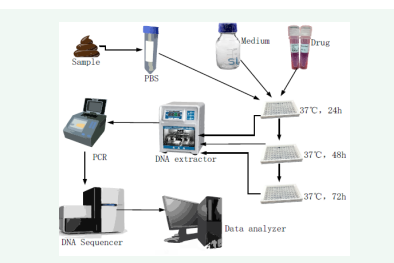
On the basis of the above-mentioned individual bacteria test, the method on the mixed intestinal bacteria was designed [Figure 3].
Figure 3: Flow chart of testing drugs on gut microbiota.
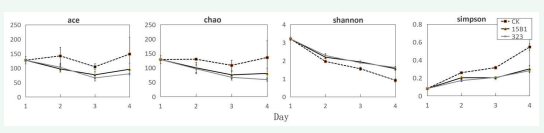
The effects of Pichia fermentans 15B1 and Pichia fermentans 323 on intestinal microbiota were then tested using this method. After DNA sequencing, it can be found that in terms of microbiota diversity, the number of bacteria in the control sample was basically stable within 4 days according to Ace and Chao values, but the Shannon index and Simpson index revealed that its diversity was significantly decreased gradually. The effects of P. fermentans 15B1 and P. fermentans 323 strains on the diversity of intestinal bacteria were similar [Figure 4].
Figure 4: Effects of strains 15B1 and 323 on bacterial diversity
Compared with the control, their diversity were significantly improved according to Shannon index and Simpson index although the number of bacteria decreased gradually over time and decreased significantly on the fourth day. The addition of P. fermentans was obviously beneficial to promote the diversity of intestinal microbiota.
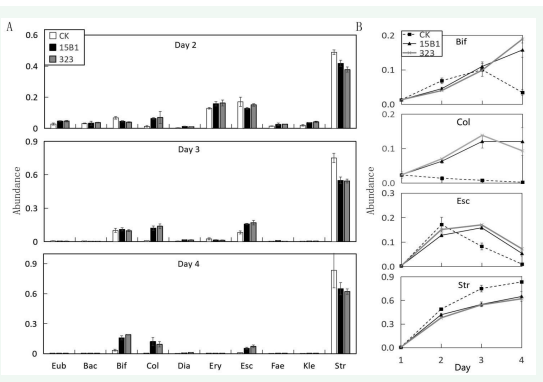
Further analysis to the main 10 genera showed that the overall change of intestinal microbiota induced by P. fermentans 15B1 and P. fermentans 323 were similar, but there were some significant differences compared with the control [Figure 5A].
Figure 5: Distribution of main genera of gut microbiota under different treatments. A. The ten genera with the largest abundance in the 2nd-4th days. B. The abundance variation of four most obvious genera in each treatment. Eub: Eubacterium, Bac: Bacteroides, Bif: Bifidobacterium, Col: Collinsella, Dia: Dialister, Ery: Erysipelotrichaceae, Esc: Escherichia, Fae: Faecalibacterium, Kle: Klebsiella, Str: Streptococcus
Taking the changes of Bifidobacterium, Collinsella, Escherichia and Streptococcus with the highest abundance within 4 days as an example [Figure 5B], Bifidobacterium in the control group increased gradually in the first 3 days, decreased sharply on the 4th day, and fell back to the original level, indicating that the medium environment was very unfavorable to Bifidobacterium. In the environment containing P. fermentans fermentation broth, Bifidobacterium was similar to the control in the first 3 days and gradually increased, but it did not decrease on the 4th day but continued to increase. The final average abundance was significantly higher than that of the control. The average abundance of 15B1 group was 15.8 %, which was 4.6 times the control and 12 times its own at 1d. The average abundance of 323 group was 18.9 %, which was 5.6 times the control and 14.4 times its own at 1d, indicating that P. fermentans 15B1 and P. fermentans 323 could significantly promote the growth of Bifidobacterium. For Collinsella, the control group decreased gradually in 1-4d, while 15B1 and 323 groups increased rapidly in 3d, but tended to be stable on the 4th day, and the final average abundance was significantly higher than that of the control group. The average abundance of 15B1 group was 12.0 %, which was 54.9 times the control group, and the average abundance of 323 group was9.3 %, which was 42.5 times the control group. For the genus Escherichia, the control group reached a maximum abundance of 17.1 % on the 2nd day, and then continued to decline. On the 4th day, it decreased to 1 %, only slightly higher than the 0.3 % of the initial 1d. The 15B1 and 323 groups reached the maximum abundance of 15.8 % and 17.0 % on the 3rd day, respectively, and then decreased to 5.3 % and 7.1 % on the 4th day, respectively. For Streptococcus, the overall trend of abundance in the control group, 15B1 group and 323 group increased with the increase of days, reaching 8.4 %, 6.5 % and 6.2 % after 4 days, respectively, which were 187.9 times, 146.2 times and 139.8 times their own at 1d, indicating that the culture environment of the three groups was more favorable for the growth of Streptococcus, and the effect of P. fermentans was not significantly different from that of the control.
DISCUSSION
The Advantages of this Method
For the cultivation of intestinal bacteria and drug testing,anaerobic conditions are the key to its success, because most anaerobic bacteria cannot survive at daily oxygen concentrations. Even for some facultative anaerobic bacteria present in intestinal bacteria, such as Escherichia coli, their metabolic patterns under aerobic and anaerobic conditions are also different, and their responses to drugs are also different [10]. The characteristic of this method was to control the anaerobic environment within a small range, which greatly reduced the cost and improved the convenience. In order to achieve anaerobic purposes, the experimental device first used a multi-layer liquid seal to isolate the culture medium. The bottom layer of silicone oil had the effect of defoaming in addition to isolation. The upper layer of silicone oil was Ve, which not only played a role in isolating the culture but also gradually consumed oxygen. This design reduced the burden of deoxidizers around microplate, and made large-scale and fast deoxygenation of environment no longer necessary for the culture of anaerobic strains.
Previous studies have shown that many anaerobic bacteria can be cultivated under aerobic conditions by adding multiple deoxidizers such as ascorbic acid, glutathione and uric acid to media [15]. Although this method is helpful for separating and enriching certain types of anaerobic bacteria, the addition of additional deoxidizers may interfere with the effects of various drugs on the growth of intestinal bacteria. Obviously, this method only used simple isolation and small-scale deoxygenation, and the medium involved was consistent with that under anaerobic workstation. Therefore, the results of growth detection in our method are more universal and comparable. In addition, this method can be conveniently operated in a biosafety cabinet. Compared to safety protection in anaerobic incubators, the safety and operating space of this method are also more easily guaranteed.
Although the growth of different anaerobic bacteria varied greatly, it proved to be feasible to detect the growth of anaerobic bacteria by microplate readers using the method. For example, the detection of this method found that the growth of Bifidobacterium was slow and it was still in a slow rising stage after 18 h. Clostridium butyricum in this study grew very fast and can reach a stable phase in about 2h, which can be used to test the effect of drug quickly. However, it was difficult to measure the absorbance due to the plenty of gas produced. In this case, silicone oil can play an important role in ensuring the stability of absorbance when detecting bacterial growth. In addition, the bacterial suspension will inevitably be briefly exposed to the air to cause a decrease in activity during the daily transfer culture. At this time, the ice bath was very necessary. The ice bath made the microorganisms in a short period of dormancy, avoiding oxygen damage, and the ice bath also made the growth of each culture hole more consistent.
When comparing the effects of drugs in this method, error control within groups is crucial for finding significant increase or decrease of some bacteria after treatments. From the detection process of gut microbiota in this study, the intra-group error mainly included the difference of bacterial growth, the difference of DNA extraction and the difference of sequencing. For the difference of bacterial growth, it was controlled by ice bath. The results from growth curve indicated that the growth of bacteria in same group had a good consistency. For the error caused by DNA extraction and sequencing, it is common in the statistical analysis of bacterial diversity. We tested it by dividing the fecal sample into three parts to extract DNA and sequencing (i.e., the error data of sample 1d). It was found that most of the errors of bacteria with abundance above 1% were controlled within 10%, which was equivalent to the error of OD600 from single bacterial growth curve among the wells. In fact, although the existence of these errors will reduce the resolution of distinguishing effects caused by different treatment, the different effect will be amplified by transfer culture of several days. For example, the increase of Bifidobacterium by Pichia pastoris 15B1 treatment was not very obvious in the first several days, but there was a significant difference between the fourth day and the control.
The Improvement of this Method
Presently, this method used disposable microplates made of polystyrene PS which have good light transmittance but have certain permeability to oxygen. To solve this problem, the quartz microplates was being used in recent experiments, which further enhanced the effect of isolating oxygen. Moreover, the quartz material is transparent to ultraviolet light and has a good effect on the monitoring of specific substances in microbiota sample. For example, 290 nm can obtain more accurate results of cell growth, which may be related to the content of protein and nucleic acid [16]. In addition, there are many types of anaerobic bacteria, and this method only tested three strains individually. More strict anaerobic bacteria need to be added in the future, especially some bacteria that are extremely sensitive to oxygen and do not produce spores, such as Roseburia and Blautia [17].
There is still much room for improvement in the detection of bacterial species and functions. For example, in terms of species classification, short fragments of 16s rRNA are currently sequenced and sequencing accuracy can only reach genera with 97 % similarity. In the future, it can be deepened to the level of species by increasing the length of 16s rRNA sequencing, and even diversity analysis on functional gene sites [18]. Furthermore, on the basis of species identification, metabolome analysis can be combined to obtain more valuable information on microbiota.
The base medium we used presently based on the appropriate adjustment of YCFA [14]. From the perspective of the bacterial diversity, the bacterial diversity of sample in the control medium reduced gradually, suggesting that the culture conditions need improvement to simulate the in vivo environment [19]. In addition, when applying this method to detect the impact of nutrients on gut microbiota, a series of simulated digestion pretreatments should be considered before adding these drugs into microplates. At present, only one sample of gut microbiota from one volunteer was used for the detection of different drugs. In the future, this method can be applied to multiple samples from different persons to support the development of precision nutrition.
ACKNOWLEDGMENTS
This work was supported by Key Scientific and Technological Grant of Zhejiang for Breeding New Agricultural Varieties (2021C02066-12) and Projects of Zhejiang Science and Technology [2024F1065-3]. In addition, we would like to thank Dr. Lei Gao and Associate Prof. Qunying Jin for the support of materials and methods.
CONTRIBUTION OF AUTHORS
H.Z.P and T.J.Z contributed to the experimental design. Material preparation, data collection and analysis were mainly performed by X.Y.L and X.F.L.
REFERENCES
- Mauerhofer LM, Pappenreiter P, Paulik C, Seifert AH, Bernacchi S, Rittmann S. Methods for quantification of growth and productivity in anaerobic microbiology and biotechnology. Folia Microbiol (Praha). 2019; 64: 321-360.
- Maier L, Pruteanu M, Kuhn M, Zeller G, Telzerow A, Anderson EE, et al. Extensive impact of non-antibiotic drugs on human gut bacteria. Nature. 2018; 555: 623-628.
- Hungate R. A roll tube method for cultivation of strict anaerobes. Methods Microbiol. 1969; 3: 117-132.
- O’Brien JP, Malvankar NS. A Simple and Low-Cost Procedure for Growing Geobacter sulfurreducens Cell Cultures and Biofilms in Bioelectrochemical Systems. Curr protoc Microbiol. 2016; 43: A.4K.1- A.4K.27.
- Speers AM, Cologgi DL, Reguera G. Anaerobic cell culture. Curr Protoc Microbiol. 2009: Appendix 4: Appendix 4F.
- Hong W, Rao FQ, Zhao XX, Guo ZY, Chen YM, Wang B, et al. An inexpensive anaerobic chamber for the genetic manipulation of strictly anaerobic bacteria. Anaerobe. 2021; 69: 102349.
- Saha US, Misra R, Tiwari D, Prasad KN. A cost-effective anaerobic culture method & its comparison with a standard method. Ind J Med Res. 2016; 144: 611-613.
- Arain S, Weiss S, Heinzle E, John GT, Krause C, Klimant I. Gas sensing in microplates with optodes: Influence of oxygen exchange between sample, air, and plate material. Biotechnol Bioengin. 2005; 90: 271- 280.
- Koutny M, Zaoralkova L. Miniaturized kinetic growth inhibition assay with denitrifying bacteria Paracoccus denitrificans. Chemosphere. 2005; 60: 49-54.
- Eini A, Sol A, Coppenhagen-Glazer S, Skvirsky Y, Zini A, Bachrach G. Oxygen deprivation affects the antimicrobial action of LL-37 as determined by microplate real-time kinetic measurements under anaerobic conditions. Anaerobe. 2013; 22: 20-24.
- Börner RA. Isolation and cultivation of anaerobes. Adv Biochem Eng Biotechnol. 2016; 156: 35-53.
- Chauvet AAP, Agarwal R, Cramer WA, Chergui M. Note: Small anaerobic chamber for optical spectroscopy. Rev Sci Instrum. 2015; 86: 106101.
- Lin DS, Lee CH, Yang YT. Wireless bioreactor for anaerobic cultivation of bacteria. Biotechnol Prog. 2020; 36: e3009.
- Browne HP, Forster SC, Anonye BO, Kumar N, Neville BA, Stares MD, et al. Culturing of ‘unculturable’ human microbiota reveals novel taxa and extensive sporulation. Nature. 2016; 533: 543-546.
- Dione N, Khelaifia S, La Scola B, Lagier JC, Raoult D. A quasi-universal medium to break the aerobic/anaerobic bacterial culture dichotomy in clinical microbiology. Clin Microbiol Infect. 2016; 22: 53-58.
- Aijaz A, Trawinski D, McKirgan S, Parekkadan B. Non-invasive cell counting of adherent, suspended and encapsulated mammalian cells using optical density. Biotechniques. 2020; 68: 35-40.
- Pridmore A, Austin C. Variability in oxygen tolerance among bacterial strains associated with the normal intestinal microbiota. Access Microbiol. 2022; 4.
- Johnson JS, Spakowicz DJ, Hong B, Petersen LM, Demkowicz P, Chen L, et al. Evaluation of 16S rRNA gene sequencing for species and strain- level microbiome analysis. Nat Commun. 2019; 10: 5029-5011.
- Rettedal EA, Gumpert H, Sommer MOA. Cultivation-based multiplex phenotyping of human gut microbiota allows targeted recovery of previously uncultured bacteria. Nat Commun. 2014; 5: 4714-4714.