Agronomic Parameters for the Mass Production of Cuitlacoche (Ustilago maydis - Zea mays) with Male-Sterile and Commercial Maize Varieties for High Altitudes
- 1. National Autonomous University, Department of Food and Biotechnology, Mexico
- 2. Faculty of Chemistry, University City, Mexico
RESULTS
Inoculum growth
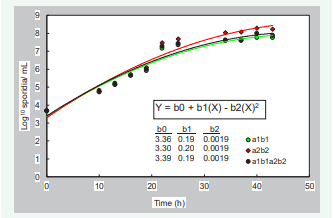
Sporidial concentration in PDB flasks increased rapidly from 8 x 103/mL (immediately after flasks were seeded), to about 5 x 107/ mL after 25 h incubation of shake cultures at room temperature. To obtain a more homogeneous variance for data analysis, sporidia concentration was log-transformed. Development of sporidia did not differ among monosporidial isolates (a1b1 or a2b2) and the mixture of compatible isolates (a1b1 + a2b2), when log10 sporidia were analyzed by covariance with isolates as a qualitative variable and time as a quantitative variable. Log growth graphs in Figure 3 are described as a quadratic function and there was no difference between the growth of pure haploid cultures (a1b1 vs. a2b2) and that of mixed cultures (a1b1 + a2b2) after comparison by covariance analysis. Noticeably, after 22 h the concentration of sporidia in shake cultures in both situations, pure haploid cultures (a1b1 vs. a2b2) or mixed cultures (a1b1 + a2b2), reached the recommended concentration of 106 to achieve good infection rates, and it did not increase substantially thereafter [Figure 3].
Figure 3: Growth of monosporidial strains (a1b1 and a2b2) of U. maydis in single and mixed cultures on PDB
Effect of time of inoculation with 15 male-sterile maize varieties
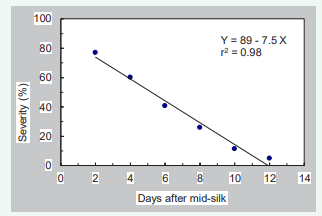
The severity of infection of maize ears by U. maydis was directly affected by inoculation time for all 15 male-sterile varieties evaluated in Champaign, IL. The most severe gall infection (80% of the ears with galls) was produced when plants were inoculated 2 days after mid-silk appearance, with a linear decrease in severity of galls that formed as inoculation time was delayed (Figure 4).
Figure 4: Correlation between severity of infection and inoculation time.
The relationship between severity of infection and time of inoculation was described by the linear regression shown in Figure 4. The severity of infection decreased about 7.5% for each day that inoculation was delayed after mid- silk emergence until less than 5% of ovaries were infected when maize ears were inoculated 12 days after mid-silk emergence .
Development of infection on the maize ears of 15 male-sterile maize varieties
The average weight of infected maize ears ranged from 470 to 735 g/ear, however, no significant differences were found among male-sterile varieties in relation to a total weight of maize ears per plot or average weight of maize ears. This lack of significant differences results from the large variability in weight of maize ears within this trial. Nevertheless, gall quality and husk protection differed significantly among varieties (Table 1).
Table 1. Husk protection and gall quality (1 to 5) for the 5 most suitable male-sterile varieties
|
Variety |
Husk protection |
Gall quality |
|
Most suitable varieties |
||
|
3153RR |
4.54 |
3.53 |
|
3356BT |
4.42 |
3.43 |
|
2730 |
4.28 |
3.00 |
|
3977 |
4.20 |
3.19 |
|
2656 |
4.19 |
2.86 |
|
Least suitable varieties |
||
|
2024 |
3.79 |
2.60 |
|
2296 |
3.68 |
2.74 |
|
1680 |
3.62 |
2.80 |
|
3028 |
3.43 |
2.47 |
|
2295 |
3.32 |
2.56 |
|
FLSD 0.05 |
0.56 |
0.51 |
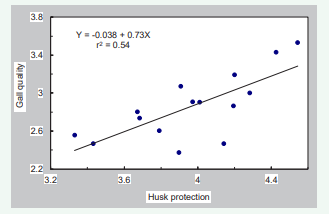
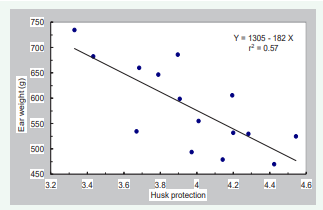
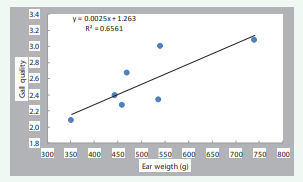
Varieties with better husk protection (3153RR, 3356BT, 2730, 3977, and 2656) were rated significantly better than those with inferior husk protection. Similarly, varieties with better gall quality (3153RR, 3356BT, and 3977) were rated higher than those with poorer quality (3028, 2295, and 2024). Husk protection was positively correlated with gall quality (r=0.73) but negatively correlated with maize ear weight (r=-0.75) (Table 2, Figures 5,6).
Table 2. Correlation among maturity, husk protection, maize ear weight and gall quality for 15 male-sterile varieties
|
|
Husk |
Ear |
Gall |
|
|
protection |
weight |
Quality |
|
Maturtity |
0.70 |
-0.48 |
0.53 |
|
Husk protection |
|
-0.75 |
0.73 |
|
Ear weight |
|
|
-0.61 |
Figure 5: Relationship between gall quality and husk protection for 15 male-sterile varieties
Figure 6: Relationship between weigh of maize ears and husk protection for 15 male-sterile varieties
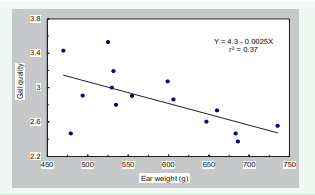
Similarly, maturity of varieties was positively correlated with husk protection and gall quality but negatively correlated with maize ear weight. This association was probably due to the good husk protection and high quality of galls on three late-maturing varieties in this trial, 3153RR, 3356BT, and 3977. These varieties appear to be well suited for the production of cuitlacoche in the USA. Although the negative relationship between maize ear weight and gall quality is perplexing (Figure 7),
Figure 7: Relationship between gall quality and weight of maize ears for 15 male-sterile varieties
it could be explained as a result of a massive infection of ovaries in early maturing maize ears. Thus, the large number of ovaries infected by U. maydis on a maize ear reduces the space available for each gall to develop into a large size, and so bad gall quality is produced.
Development of infection in seven commercial maize varieties grown in high elevation in Mexico
Six of the seven maize varieties tested in Mexico produced maize ear weights in the range of 350 to 550 g/ear, and Aspros 910 produced more than 700 g/ear (Table 3). These values are in the range of those previously obtained with the male-sterile maize varieties at the University of Illinois. Regarding gall quality and husk protection, Aspros 910 was among the best-ranked varieties and was superior to other varieties concerning the weight of galls (326.6 g), i.e., the commercial cuitlacoche product. The average weight of maize ears and galls produced on ears of Aspros 910 were similar to those reported by Valdez-Morales et al. [12], for other maize varieties evaluated in northern Mexico. A preliminary estimation indicates that Aspros 910 could be used to produce commercial yields of 15 tons/ha of cuitlacoche galls.
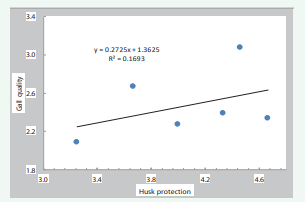
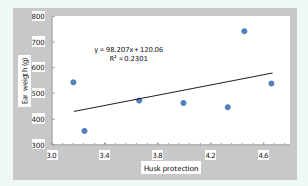
Some differences were observed when comparing results obtained with the seven maize varieties grown at high elevation in Mexico with those obtained for the 15 male-sterile maize varieties evaluated in Illinois. With the Mexican maize varieties, husk protection correlated positively with both gall quality (r=0.41) and maize ear weight (r=0.47) (Figures 8,9),
Figure 8: Relationship between gall quality and husk protection in 7 maize varieties for high valleys (Mexico).
Figure 9: Relationship between weight of maize ears and husk protection in 7 maize varieties for high valleys (Mexico)
in contrast to the negative relationship observed between maize ear weight and husk protection with the male-sterile maize varieties evaluated in Illinois (Figure 6). The relationship between gall quality and maize ear weight was also different, as positive correlation was observed for the maize varieties evaluated in Mexico; i.e., the best quality galls were produced on ears that had greater weights (Figure 10),
Figure 10: Relationship between gall quality and weight of maize ears in 7 maize varieties for high valleys (Mexico)
but a negative correlation was observed with the male-sterile maize varieties, i.e., the best gall quality was obtained from maize ears of lower weight (Figure 7).
Significant differences regarding maturation time of plants (presence of tassels) and ripening time of galls (gray-silver, without the peridium breaking) were also observed among the seven maize varieties evaluated at high elevation in Mexico. Maize ear size was positively correlated with maize ear weight and gall weight. However, none of these variables correlated with gall quality; i.e., size of galls. In this set of experiments, varieties Aspros 910, Aspros 722, and Aspros 720 had the best overall agronomic characteristics (Table 3), and are good candidates for mass production of cuitlacoche in Mexico.
Table 3. Agronomic characteristics and development of infection with U. maydis in 7 maize varieties for high valleys (Mexico)
|
:: |
Time for formation of structures (days) |
|
Weight (g) |
|
|
|
|
||
|
Maize variety |
after planting |
after inoculation |
Maze year Size(cm) |
|
|
|
|
|
|
|
|
tassel |
silk |
galls |
|
Infected maize ear |
Galls |
Severity of infection (%) |
Husk protection |
Gall quality |
|
Aspros 910 |
68 ± 1.3a |
73 ± 1.7a |
22 ± 1.3a |
25.9 ± 1.4b |
740.9 ± 113.5d |
326.6 ± 79.2c |
97.9 ± 2.4a |
4.5 ± 0.5de |
3.1 ± 0.5c |
|
Aspros 720 |
116 ± 1.8d |
117 ± 0.5e |
32 ± 0.5c |
22.4 ± 1.7a |
536.6 ± 36.6c |
285.0 ± 83.6bc |
98.9 ± 3.3a |
4.7 ± 0.5e |
2.3 ± 0.5ab |
|
Grano de Oro |
106 ± 1.8d |
107 ± 1.8d |
22 ± 2.0a |
22.8 ± 2.9a |
444.6 ± 69.9b |
269.1 ± 60.7b |
98.5 ± 2.7a |
4.3 ± 0.6de |
2.4 ± 0.6ab |
|
Aspros 722 |
83 ± 1.1b |
84 ± 1.1b |
26 ± 0.5b |
25.0 ± 2.8b |
541.2 ± 83.6c |
256.5 ± 40.1b |
96.5 ± 5.1a |
3.2 ± 0.4a |
3.0 ± 0.8c |
|
Cronos |
103 ± 3.4c |
105 ±I 4.0c |
21 ± 2.0a |
22.2 ± 2.8a |
470.4 ± 89.5bc |
251.5 ± 60.9b |
99.0 ± 1.8a |
3.7 ± 0.6bc |
2.7 ± 0.6bc |
|
Hoja de Plata |
104 ± 3.9c |
105 ± 3.6c |
22 ± 1.8a |
23.2 ± 2.4a |
460.1 ± 85.3b |
246.1 ± 52.6b |
99.6 ± 1.9a |
4.0 ± 0.6cd |
2.3 ± 0.5ab |
|
Vulcano |
103 ± 4.6c |
104 ± 4.6c |
21 ± 2.4a |
21.3 ± 2.7a |
351.6 ± 87.4a |
169.6 ± 48.6a |
98.1 ± 3.2a |
3.3 ± 0.6ab |
2.1 ± 0.3a |
*Different letters indicates statically significant difference between the values of different samples in column.
DISCUSSION
Sporidial concentration in inoculum of U. maydis has been reported to affect both, severity of infection (percentage of the maize ear covered with galls) and incidence (the number of of ears with galls), i.e., du Toit and Pataky [1] reported a 40% increase in incidence of infection when inoculum concentration was increased from 103 to 106 sporidia/mL. Accordingly, a concentration of 105 to 106 sporidia/mL has been recommended by several authors [2-5,12,18]. According to the initial experiments, sporidial concentrations of 106 sporidia/mL were obtained after 24 h incubation, thus maize plants were inoculated in all experiments with U. maydis cultures incubated for 24 h .
In greenhouse preliminary experiments, sporidial mixed cultures (a1b1 + a2b2) were used to inoculate maize seedlings. After the appearance of initial symptoms of infection, galls developed on stalks and leaves, thus suggesting that infective inoculum could be produced from heterogenic sporidial cultures obtained from direct germination of teliospores. Such inoculum (multi-teliosporic cultures) has been used in various studies [19,20,13] but infection rates (severity and incidence) were lower than those obtained when inoculum of two compatible haploid strains are used. Castañeda et al. [17], confirmed these results by comparing in a single experiment the production of galls after inoculation of maize plants with compatible haploid strains and the corresponding multi-teliosporic cultures, i.e., production of galls was significantly higher when two compatible haploid strains were inoculated. However, it would be necessary to conduct more experiments to test different compatible haploid strains and multi-teliosporic strains to inoculate different maize cultivars, either as single cultures or in combinations to evaluate their real infective capacity.
With the 15 male-sterile maize varieties evaluated in Champaign IL decreasing infection by U. maydis was observed as inoculation time progressed from 2 to 12 days after mid-silk emergence. Two interrelated explanations can be given for this observation; silk “aging” [8,9,18] might result in the inability of the fungus to complete infection on older silks; however, the effect of pollination could be an alternative explanation on inability of the fungus to cross from the silk into the ovary, since an abscission zone forms at the site of attachment of the silk to the ovary once pollination of the ovary has occurred [7], thus creating a physical barrier of dead cells that inhibits infection of the ovaries by U. maydis. The high incidence and severity of galls observed with early inoculation in this study (2 days after silk appearance) (Figure 4) differed from results reported previously, when good infections were obtained with maize plants inoculated 4-8 days after mid-silk emergence [2-5,10,12,14,21]. Pataky and Chandler [10] stated that a large percentage of maize ears were not infected with early inoculation of maize varieties, i.e., 2 days after mid-silk emergence. The authors proposed that some silks had not yet emerged from the cobs and recommended that inoculation of maize ears 4-6 days after mid-silk emergence is the best timing for high galls yields. Various explanations can be given for the differences observed in those studies, compared with the results obtained in our study. Varieties of maize respond differently regarding emergence and maturation of silks, a difference reported by Pataky and Richter [11] about the time of senescence of silks affecting the response to U. maydis. Other aspects of maize floral development might also result in large differences in silk emergence and pollen production. Quick abscission of the silks following pollination might also reduce the number of galls produced by minimizing the period that ovaries on maize ears are exposed to infection by U. maydis. To guarantee efficient production of cuitlacoche, it is of utmost importance to know the phenology (i.e., emergence and senescence of silks) of the varieties of maize selected for producing galls. Furthermore, environmental conditions (humidity and temperature) and their influence on the flowering processes of the plant have to be considered in order to establish the most appropriate time of inoculation for each maize variety.
Maize ears that weighed more tended to produce many small galls whereas those with lower weight produced a reduced number of larger galls. Possibly, this relationship was due to the number and size of kernels per maize ear, which varied among varieties. Varieties with fewer but larger galls perhaps would be more suitable for commercial production of cuitlacoche than those with many small galls. In addition, this phenomenon is also probably related to the effectiveness of inoculation, since ears with many infected ovaries (high severity of infection) have limited space between galls, blocking the development of large galls. This phenotypic characteristic is very important from a commercial perspective because consumers prefer large galls, i.e., those usually found on naturally infected ears of maize.
From a commercial perspective, the Mexican maize varieties used in the experiments in Mexico showed phenotypic characteristics of marketable interest; i.e., the positive relationship between gall quality, the weight of maize ears, and good husk protection represent added value in comparison to galls produced on the USA male-sterile maize varieties. For the experiments carried out in Mexico, the best inoculation time obtained with the male sterile varieties at Illinois, i.e., two days after mid silk emergence, resulted in more yield of galls despite the recommendations for a later inoculation time [10]. This was confirmed by the high severity of infection obtained with all maize varieties tested in Mexico, > 96% for all varieties tested (Table 3), while other authors attempting commercial production of cuitlacoche reported lower severities when using later inoculation time [6,12,19]. This is an additional factor of importance for the mass production of cuitlacoche, which was probably favored by regular detasseling of maize plants. Pollination of ovaries is hence reduced by this procedure and, by inoculating before pollination and senescence of silks, high severity and incidence of smut galls is likely to be guaranteed with almost any variety of maize.
Since cuitlacoche is a traditional food consumed in Mexico, the taste, mainly sweetness, and bitterness, is a key factor for commercial acceptance by consumers. Preliminary consumer trials with cuitlacoche galls obtained with the best producing variety in Mexico, i.e., Aspros 910, revealed a rather bitter taste. Thus, further tests with other maize varieties should be performed and sensory profiles developed for galls produced on diverse maize cultivars. Taste, agronomic characteristics (weight and size of galls), are critical parameters to select the most suitable varieties for commercial production of cuitlacoche [17-22].
CONCLUSION
Inoculum of the common smut fungus, U. maydis, can be produced from compatible monosporic strains, using a concentration of sporidia of 106 per mL for successful infection. Severity of infection above 96% was obtained by inoculating maize ears 2 days after mid-silk appearance. As demonstrated in many studies, silk age and pollination influence the effectiveness of inoculation for production of cuitlacoche.
The field studies provided evidence that maize varieties might differ in response to infection by U. maydis, depending on their genotype, seasonal and environmental conditions. Therefore, careful selection of maize cultivars must be carried out for a successful mass production of cuitlacoche galls throughout the year.
The results obtained with the commercial maize varieties adapted to production in high altitudes in Mexico indicate that, for production of this gourmet edible fungus in Mexico, local maize varieties have to be tested to select for each particular location and identify varieties with relevant agronomic characteristics, i.e., weight and gall quality, short overall time for formation of galls after planting, and suitable flavor (sweetness vs. bitterness). This approach will help to fully develop this new agro-industrial activity of commercial-scale cuitlacoche production in Mexico.
ACKNOWLEDGMENT
Authors thank Dr. Jerald Pataky (Department of Crop Science University of Illinois) for his valuable help in providing facilities, U. maydis strains, maize male-sterile plants, and assessment for the tests carried out at the University of Illinois by Castañeda de León
REFERENCES
- du Toit LJ, Pataky JK. Variation associated with silk channel inoculation for common smut of sweat corn. Plant Dis. 1999; 83: 727- 732.
- Thakur RP, Leonard KJ, Pataky JK. Smut gall development in adult corn plants inoculated with Ustilago maydis. Plant Dis. 1989; 73: 921- 925.
- Pataky JK. Production of cuitlacoche [Ustilago maydis (DS) Corda] on sweet corn. Hort Sci. 1991; 26: 1374-1377.
- Pope DD, McCarter SM. Evaluation of inoculation methods for inducing common smut of corn ears. Phytopathol. 1992; 82: 950-955.
- Snetselaar KM, Mims CW. Infection of corn stigmas by Ustilago maydis: Light and electron microscopy. Phytopathol. 1993; 83: 843-850.
- Villanueva C, Sánchez E, Villanueva E. El huitlacoche y su cultivo. Mundi Prensa México, S.A. de C.V. 2007.
- Snetselaar KM, Carfioli MA, Cordisco KM. Pollination can protect maize ovaries from infection by Ustilago maydis, the corn smut fungus. Can J Bot. 2001; 79: 1390-1399.
- Bassetti F, Westgate ME. Emergence, elongation, and senescence of maize silks. Crop Sci. 1993; 3: 271–275.
- Bassetti F, Westgate ME. Senescence and receptivity of maize silks. Crop Sci. 1993; 33: 275–278.
- Pataky JK, Chandler MA. Production of huitlacoche, Ustilago maydis: timing inoculation and controlling pollination. 2003; 95: 1261-1270.
- Pataky JK, Richter PM. Silk abscission in two sweet corn (Zea mays L.) hybrids that differing susceptibility to common smut infection of ears. Hort Sci. 2007; 42: 1409–1412.
- Valdez-Morales M., Valverde M.E., Paredes-López O. Procedimiento tecnológico para la producción masiva de huitlacoche.http://octi. gunajuato.gob.mx/sinnco/formulario/MT/MT2009/MT1/SESION2/ MT127 de febrero 2011. 2009
- Escalante-Pérez C, Villanueva-Sánchez E, Sahagún-Castellanos J, Sánchez-Cabrera I, Villanueva-Verduzco C. Dynamics of huitlacoche harvest (ustilago maydis cda.) in nine commercial corn hybrids. Tropical Subtropic Agroecosys. 2020; 23: 3
- Pataky JK. Production of huitlacoche (Ustilago maydis) using inoculation techniques developed to screen reactions of sweet corn to common smut. In: Sánchez JE, Huerta G, Montiel E. Editors Procedures of the 4th International Conference Mushroom Biology and Mushroom Production. Cuernavaca. Mexico. 2002; 31-41.
- Pope, D.D, Pataky JK. Inoculation techniques to produce galls of common smut on ears of sweet corn. Phytopathol. 1992; 82: 950-995.
- Valverde ME, Moghaddam PF, Zavala Gallardo MS, Pataky JK, Paredes López O, Pedersen WL. Yield and quality of huitlacoche on sweet corn inoculated with Ustilago maydis. Hort Sci. 1993; 28: 782-785.
- Castañeda de Léon V, Martínez-Carrera D, Morales P, Sobal M, Gil- Muñoz A, Severiano-Perez P, et al. Productivity and flavor of diverse genotypes of Ustilago maydis “cuitlacoche” for human consumption. Fungal Biol. 2019; 123: .481-488.
- Lindsey J. du Toit, Jerald K. Pataky. Effects of silk maturity and pollination on infection of maize ears by Ustilago maydis. Plant Dis. 1999; 83: 621-626.
- Martínez ML, Villanueva VC, Sahagún CJ. Susceptibilidad y resistencia del maíz al hongo comestible huitlacoche (Ustilago maydis Cda.) mejorando su resistencia. Revista Chapingo Ser. Hortic. 2000; 6:241- 255.
- Aydo?du M. Huitlacoche yield in some maize varieties in the Mediterranean region of Turkey. Food Sci. Technol. 2015; 35, 386- 390.
- Pataky JK, Nankam C, Kerns M. Evaluation of a silk-inoculation technique to differentiate reactions of sweet corn hybrids to common smut. Phytopathol. 1995; 35:1323-1328.
- Castañeda de León VT, Leal Lara H. Logros y desafíos de la producción masiva de cuitlacoche Ustilago maydis en México. In “Hongos comestibles y Medicinales en Iberoamérica: investigación y desarrollo en un entorno multicultural”. Sánchez J.E., Mata G. (Eds.) El Colegio de la Frontera Sur, México. 2012; 193-206.
Abstract
In the central region of Mexico, maize ears with galls resulting from infection by the fungal pathogen Ustilago maydis, known as cuitlacoche, are consumed as a traditional food. In initial experiments (performed in Champaign, Illinois, USA), male-sterile maize varieties were used to evaluate factors affecting controlled production of galls, such as production of inoculum and timing of inoculation in relation to maturity of the ears. Characteristics of the infected ear like husk protection, the weight of the infected ears, severity of infection and quality of galls were also rated in these experiments. In a second set of experiments in Guerrero (Mexico) seven commercial maize varieties were tested and additional agronomic parameters like the phenological characteristics of the inoculated plants (the size of maize ears and the weight of galls) were also recorded. High infection rates were obtained throughout the study by inoculating mixtures of two compatible monosporidial strains of U. maydis with mating type a1b1 and a2b2. In the experiments carried on in Champaign, Illinois, it was stablished that 2 days after silk emergence was the optimum inoculation time, and while the total weight of galls on maize ears ranged from 470 to 735 g/ear, male-sterile varieties 3153RR, 3356BT and 3977 were the highest yielding strains. Furthermore, differences in gall quality and husk protection were observed among male-sterile maize varieties. In the experiments with commercial maize varieties for high altitudes in Pilcaya, Guerrero (Mexico), Aspros 910 and Aspros 722 produced the highest yields (740 and 541 g/ear) with a short growing cycle (73 and 84 days to silk formation) and good gall quality (3.5 cm diameter). These experiments showed that the selection of a maize variety is an important factor for the commercial production of cuitlacoche since it strongly influences ear weight, husk protection, gall quality and the length of the growing cycle.
Keywords
• Year-round production of cuitlacoche
• Maize ears with galls
• Inoculum parameter
• Susceptible maize lines
• Commercial characteristics of galls
CITATION
de León VC, Lara HL (2024) Agronomic Parameters for the Mass Production of Cuitlacoche (Ustilago maydis - Zea mays) with Male-Sterile and Commercial Maize Varieties for High Altitudes. J Hum Nutr Food Sci 12(3): 1188.
INTRODUCTION
To develop an efficient production system for cuitlacoche, an edible delicacy comprising the galls produced on maize ears infected by the common smut fungus, Ustilago maydis, the host- parasite cycle should be understood effectively. Cuitlacoche develops as a response of maize to infection following inoculation of two monosporidial compatible strains (i.e., a1b1 and a2b2) of Ustilago maydis. Many factors can influence the development of infection, including: concentration of inoculum, the timing of inoculation, silk maturation and abscission, plus pollination. Maximum control of these variables is necessary to achieve high rates of infection and, thus, produce high-quality cuitlacoche galls (mature tumors filled with teliospores resulting from infection of the ovaries by U. maydis). Du Toit and Pataky [1] found that the incidence of galls raised by more than 40% when the concentration of inoculum was increased from 103 to 106 sporidia/mL. In fact, several authors [1-6] agree that efficient inoculation is assured with 105 to 106 sporidia/mL.
Infection of maize by U. maydis occurs when two genetically compatible monosporidial strains come into contact on the plant surface. After recognition of sexual compatibility, the sporidia fuse to form a dikaryotic mycelium, which is the infectious stage of U. maydis. This mycelium penetrates the stigma or silk and moves down to infect the ovary. This event competes with pollination as when a pollen grain germinates on the silk and the pollen germ tube migrates all the way down the silk to the ovaries, resulting in their fecundation. Then, an abscission zone is formed at the base of the silks of the pollinated ovaries, which acts as a physical barrier that prevents the infective mycelium of U. maydis from entering the ovary [7].
The age of the silks and the time of inoculation is a critical factor influencing the severity of infection (the percentage of the maize ear covered with galls). Bassetti and Westgate [8, 9], reported that the silks are no longer receptive to infection by the common smut fungus for 4 days after emergence of silks in some maize varieties, but with other genotypes, receptiveness to infection is maintained up to 10 days. To ensure high infection rates of maize ears by U. maydis, inoculation should be performed during silk emergence, and prior to pollination [10,11]. The length of silks (3-5 or 5-10 cm) appearing at the top of the maturing ear has been proposed as a criterion for inoculation by some authors [6,12,13]. Since all silks do not emerge from the husk leaves at the same time (i.e., the silks emerging first are those being formed at the bottom of the ear) the length of the silks is associated with the stage of development in the maturing maize ear. A short length indicates that a large proportion of the ear is in an initial stage of silk formation and then it may be more receptive to infection by U. maydis. Pataky [14] indicated that this criterion is influenced by different factors, there is a high variability in the development of maize plants of the same cultivar, a high variation in the viability of silks among different maize cultivars and even within the same variety of maize. A critical factor to consider is that pollination greatly determines receptiveness of silks to infection by U. maydis, so inoculation of ears at early maturing stage is a strategy to diminish the pollination effect. Based on other criterion, various authors reported good infection rates by inoculating compatible sporidial strains of U. maydis 5 days after the first emergence of silks [3-7,15,16].
Pataky and Chandler [10] experimented with two sweet maize hybrids and three male-sterile maize hybrids in order to elucidate the influence of pollination and inoculation time on severity of infection by U. maydis. Plants were inoculated 2 to 12 days after mid-silk emergence. With sweet maize cultivars, the highest severity of infection was observed when inoculation took place 4 days after mid-silk emergence, and the severity was greater on detasseled (70%) than on pollinated plants (50%) with male- sterile hybrids, maximum severity (55%) was observed when plants were inoculated 6 days after mid-silk emergence and no galls were produced if plants were pollinated by hand prior to inoculation. In all cases, severity of infection decreased when plants were inoculated earlier, 2 days after mid-silk emergence, or if inoculation was delayed for 8 or more days. In relation to the yield of edible product, i.e., cuitlacoche (galls), the highest yields (weight of cuitlacoche galls /ear) were produced when plants were inoculated 6 days after mid-silk emergence. With sweet maize, an average yield of 92 g/ear was obtained with detasseled plants, and yield diminished to 78 g/ear on non- detasseled plants. Noticeably, male-sterile hybrids produced greater yields, 131 g/ear, but gall yield decreased to 100 g/ear when plants were pollinated. With both types of hybrids, greater yields of cuitlacoche and severity of infection were closely associated.
A sound understanding of the host-parasite interaction is, therefore, needed to develop a process for controlled production of cuitlacoche, instead of the seasonal collection of galls from naturally infected maize plants. To achieve high infection rates by U. maydis, critical parameters have to be evaluated like maize plant-fungal parasite interaction, mass production of inoculum, stage of silk development, control of pollination, and inoculation time. Additionally, good agronomic procedures must be assessed for the development of healthy and strong maize plants. Furthermore, maize cultivar genotype and phenotype must be considered since they also influence the course of infection by U. maydis to produce cuitlacoche galls that match consumer preferences (i.e., galls size and weight, flavor, color and texture of galls) [17].
MATERIALS AND METHODS
Ustilago maydis strains and preparation of inoculum
Compatible monosporidial (haploid) strains of U. maydis (a1b1 and a2b2) were provided by Dr. Pataky (University of Illinois), that originally were isolated and designated isolates 2 and 11 by Dr. K. Leonard [U S. Dept. of Agriculture Agricultural Research Service (USDA-ARS) University of Minnesota, St. Paul].
Monosporidial (haploid) strains reproduce by budding; they were grown on 15 mL potato-dextrose agar (PDA) plates, which were stored at 20C and sub-cultured every 4 months. To evaluate growth of isolates in shake cultures, two replicates of each of the two individual isolates (a1b1 or a2b2) and a mixture of the both isolates (a1b1 + a2b2) were incubated in 500 mL sterile potato- dextrose broth (PDB) at room temperature and 120 rpm. Flasks with PDB were each inoculated with a piece of agar (1 cm x 1 cm) colonized by sporidia. A 5 mL aliquot was sampled from each flask every 3 h, from 9 to 43 h after inoculation. The number of sporidia per mL was counted for each sample using a hemocytometer. The growth of each monosporidial isolate and the mixture of isolates were compared by covariance analysis. Sporidial growth rate was monitored in shake cultures containing individual monosporidial isolates and a mixture of two compatible isolates in order to determine if inocula could be eventually produced directly from teliospores (from which mixtures of compatible haploid sporidia would arise). The experiment was done twice. For plant infection experiments, inoculum was produced from 24 h broth cultures of each haploid strain types (a1b1 and a2b2) that were grown separately. Equal volumes of each culture were mixed and then diluted in 10 parts of distilled water, so the resulting suspension with 106 sporidia/mL served as inoculum for infection of corn seedlings in greenhouse preliminary experiments and for maize plants in field.
Time of inoculation and agronomic characteristics of galls of male- sterile maize varieties
Two field studies were performed at the University of Illinois South Farms (Champaign) USA (40° 06’ 35”N 88° 12’ 15”E), 226 m above sea level with an average annual rainfall of 966 mm, representing a warm and temperate climate. The statistical design was a randomized complete block. Each field study included four independent experimental replicates of 15 male-sterile maize varieties (1680, 2024, 2295, 2296, 2540,2550, 2646, 2656, 2680, 2730, 2760, 3028, 3153RR, 3356Bt, and 3977). Each experimental replicate was a plot with 4 rows. Each row with about 22 plants was 5.5 m long. In the first field study, plants were inoculated 2, 4, 6, 8, 10, and 12 days after mid-silk emergence (50% of plants with silks emerged in a plot) with a mixture of compatible U. maydis isolates (a1b1 and a2b2). A volume of 6 mL sporidial suspension was injected down the silk channel with an automatic syringe connected to a plastic bottle with a hose [the same equipment used by Valverde et al. (16)]. In the second field study, plants were inoculated 2 days after mid- silk emergence to evaluate the agronomic characteristics of galls (i.e., severity of infection, husk leaves protection and quality of galls). In both field studies at the University of Illinois, primary maize ears were harvested 19 days after inoculation, from 12 plants in the central 2 rows of each lot of 4 rows per plot. Each maize ear was weighed, severity of infection was measured for all primary maize ears in a row, and the presence of galls on maize ears was rated for each ear on a scale of 0 to 100% in increments of, 25, 50, 75, and 100%. Protection of the galls by the husk leaves was rated from 1 to 5 for each maize ear, i.e., 1 corresponded to a maize ear without husk leaves, 2 equated to 50% coverage of the ear with husk leaves, 3 was equal to 70% coverage by husk leaves, 4 was equivalent to 90% of the ear surface covered by husk leaves, and 5 to complete coverage of the ear with husk leaves (Figure 1).
Figure 1: Protection on maize ears (husk protection) with leaves, from 1 (= whole exposure) to 5 (= complete protection)
Husk leaves were removed and gall quality of the infected maize ears was rated from 1 to 5; i.e., 1 corresponded to maize ears with small galls (<1.5 cm diameter) which are inadequate for commercialization; 2 to maize ears with galls slightly larger than maize kernels (2.5 cm diameter) but too small for a good quality product; 3 to maize ears with medium-sized galls (3.5 cm diameter); 4 to ears with a mixture of small, medium and large- galls (4.5 cm diameter) and 5 to maize ears with predominantly large-galls (? 6 cm diameter), the most suitable for ideal commercialization (Figure 2).
Figure 2: Quality of infected maize ears with galls (= cuitlacoche quality): 1 (bad, galls 6 cm Ø).
Maturity of maize varieties was assessed according to the time (days) until reproductive structures were formed (tassels or silks).
Development of galls on seven commercial maize varieties grown at high altitudes in Mexico
The same basic agronomic trial set up used in the second field trial in Champaign Illinois (USA), was followed for the field study carried out in central Mexico at Pilcaya Guerrero (18° 44’ 51.8”N 99° 40’ 30.3”W), 1610 m above sea level with an average annual rainfall of 1200 to 1400 mm and a sub-tropical humid, warm climate. Seven commercial maize varieties developed for production at high altitudes (Aspros 720, Aspros 722, Aspros 910, Cronos, Grano de Oro, Hoja de Plata, and Vulcano) were tested. The field study included also four independent experimental replicates. All plants were detasseled and primary maize ears were inoculated 2 days after silk emergence. Evaluation of infection was measured by collecting infected maize ears from two to three weeks after inoculation, depending on maturation of each maize variety, and only when galls were filled with teliospores but still had an unbroken peridium. Additionally, galls from each maize ear were completely detached and weighted separately, the size of maize ears was measured, and the phenological characteristics of each maize variety were evaluated [i.e., the time (days) to tasseling of plants and the time galls were completely formed after inoculation].
All data were analyzed by analysis of variance (ANOVA) and means were compared by Waller-Duncan Bayesian minimum significant difference values, BLSD, (k = 100). Yield of cuitlacoche and severity of ear galls were compared by regression for all experiments. All analysis were done with Statical Analisis System (SAS) software (SAS Institute, Cary, North Carolina).