Effects of High Dietary Fat Intake on Biochemical Variables and Pancreas Histoarchitecture in Diabetic Rats
- 1. Department of Physiology, College of Health Sciences, Bowen University, Nigeria
Abstract
Consumption of dietary fat has been implicated in some metabolic and cardiovascular disorders of public health importance. The effects of high dietary fat intake on glycemic tolerance (GT), organ weights, lipid profile (LP) and pancreatic tissue histology were investigated respectively in 2 groups (n=8, each) of experimental male diabetic and healthy albino rats following treatment with composed high-fat diet for 6 weeks while another 2 similar groups (n=8, each) on normal diets served as normal and diabetic controls respectively in this experimentally-controlled designed study. Induction of diabetes (100mg/dL, intraperitoneally) was achieved with freshly prepared alloxan monohydrate solution after 15 hours overnight fast while oral GT test and LP analysis were conducted on blood samples. Organs were extracted and weighed while sections of pancreatic tissues histologically examined. Body weight significantly increased (P < 0.05) in both experimental healthy (40.1%) and diabetic (41.3%) rats. No significant organ weight change observed relative to high fat diet. GT improved significantly in both experimental rats with much delayed glycemic response in diabetic rats. Histopathological changes in pancreatic islets of experimental diabetic rats were observed while normal histoarchitecture and regeneration of some b cells observed in healthy and diabetic control rats respectively. Triacylglycerides (72.24±1.00mg/dL) and cholesterol (90.50±1.50mg/ dL) increased significantly in both experimental rats with higher values observed in diabetic rats. Consumption of high dietary fat in this study altered blood lipid profile with significant weight gain - a potential risk factors for metabolic and cardiovascular problems. However, consumption and recommendation of dietary fat in individuals should be rationalized to achieve positive impact on glycemic and lipid profiles
Keywords
• High-fat diet
• Glycemic tolerance
• Lipid profile
• Pancreas histoarchitecture
Citation
Chukwudike Anyakudo MM, Omotayo P (2015) Effects of High Dietary Fat Intake on Biochemical Variables and Pancreas Histoarchitecture in Diabetic Rats. J Hum Nutr Food Sci 3(1): 1053.
INTRODUCTION
Fat intake, in any of its forms, i.e., mono-unsaturated, polyunsaturated, trans and cis saturated, has long being investigated in relation to human health and a number of observational studies [1-3] have reported link between dietary fat consumption and metabolic and cardiovascular disorders. Experimental studies have shown that high-fat-induced hyperlipidemia leads to pancreatic endocrine and exocrine alterations with possible risk of pancreatic cancer [4-6]. Diabetics who have their carbohydrate intake restricted consume greater proportion of fat and such high fat intake has been linked with insulin resistance and poor glycemic control and profile [7]. The aim of diet therapy in diabetics is to achieve normoglycemia and maintain ideal body weight. In addition, dietary advice given in diabetes mellitus primarily aimed at averting symptoms of hyper- and hypoglycemia, eliminate or postpone secondary complications and normalize serum insulin concentrations, blood lipid abnormalities and blood pressure elevation. This study was carried out to determine the effects of high intake of dietary fat on glycemic tolerance (GT), organ weights, lipid profile (LP) and pancreatic tissue histology of alloxan-induced male diabetic albino rats with the rationale that findings obtained from the study may lend necessary insights to the correlation between dietary fat and glycemic control with necessary precautions and guide provided in planning, selecting and recommending dietary menu to the diabetics.
MATERIALS AND METHODS
Experimental Animals And Diets
Thirty two male albino rats weighing 200-250g were purchased from the disease-free stock of the animal house of the Department of Biochemistry, Bowen University, Iwo, Osun state, Nigeria. The rats were kept in polypropylene plastic cages and maintained at normal and standard laboratory conditions of temperature (28 ± 2 o C) and relative humidity (46 ± 6%) with 12-hour light-dark cycle and adequate ventilation for two weeks to allow for acclimatization to their environment. The rats were weighed twice weekly to ensure that no rat outside the initial weight range of 200g-250g was used. The rats were initially fed with commercially available standard rat feed (Ladokun feeds Nig. Ltd.) purchased from a commercial branch depot and water ad libitum during the period of acclimatization and thereafter were grouped into four groups: two groups (n=8, each) containing diabetic rats and other two groups (n=8, each) containing healthy rats. One of the healthy and diabetic groups respectively were fed with normal diet as controls while the remaining groups were fed with high-fat diet as experimental groups according to the experimental design for a period of 6 weeks. The weights of the rats were recorded for the six weeks period of the study prior to laboratory investigations. This study using experimental animals was conducted in accordance with the internationally accepted principles for laboratory animals [8].
Induction of Diabetes
After 15 hour overnight fast, rats in two groups were injected intraperitoneally with freshly prepared alloxan monohydrate (Sigma chemicals, USA) dissolved in sterile normal saline at a dose of 100 mg/kg body weight. Diabetes was confirmed 4-7 days later by use of glucometer (On Call Plus Blood Glucose Monitoring System, ACON Laboratories, Inc. San Diego, USA.). Rats with Fasting Blood Glucose (FBG) level > 150mg/dl were considered diabetic and used for this study.
Test Diets
The normal and the high-fat diets (Table 1) were processed and prepared by an animal science nutritionist in the department of Animal Science and Nutrition, Bowen University, Iwo, Osun State, Nigeria with the following feed compositions (% per 100g of feed):
Table 1: Percentage Compositions of normal and experimental diets.
| NUTRIENT COMPONENTS | INGREDIENTS USED | NORMAL DIET (ND) (% per 100g of feed) | HIGH FAT DIET (HFD) (% per 100g of feed) |
| Protein | Fish meal | 50% | 25% |
| Fats and oils | Groundnut cake | 35% | 60% |
| Carbohydrates | Maize | 10% | 10% |
| Fibers | Rice thusk | 0.1% | 0.1% |
| Premix (Vitamins) | Vitamins B, C, D | 0.4% | 0.4% |
| Amino acids | Lysine, Methionine | 0.2% | 0.2% |
| Water | Water | 5% | 5% |
Experimental Design
The animals after 2 weeks acclimatization period and induction were randomly divided into 4 broad categories of 8 rats each:
GROUP A: Diabetic rats on high-fat diet (DHF group)
GROUP B: Diabetic rats on normal diet (DNF group) – Diabetic control
GROUP C: Non-diabetic rats on high-fat diet (NDHF group) and
GROUP D: Non-diabetic rats on normal diet (NDNF group) – Normal control
Biochemical Assays
Oral glucose tolerance test (OGTT): The OGTT was conducted at the end of the 6 weeks of study. Animals in all groups after 15 hour overnight fast with free access to water were treated with an oral D?glucose load of 2 gm kg?1 (dissolved in distilled water) administered by means of cannula. Blood samples were withdrawn from the cordal (tail) vein of each animal (tail snipping) to determine the fasting blood sugar concentration at time 0 minute (before ingestion of glucose) and subsequently at intervals of 30, 60, 90 and 120 minutes after oral glucose administration.
Lipid profile test (LPT): The lipid profile was conducted at the beginning and then 6 weeks later at the end of the study for comparison. Blood samples from the Posterior Vena Cava vein were collected and transferred into the k3 EDTA (Ethylene Diamine Tetraacetic Acid) sample bottles. Samples were centrifuged at 3000 revolutions to obtain the plasma fractions which was kept in a refrigerator (at -70ºC) until used and the sera obtained were used for the biochemical assay of the lipid profile. Plasma concentration of total cholesterol (TC), high density lipoprotein (HDL) and Triacylglycerol (TAG) were measured by the enzymatic colorimetric method after centrifugation using a dry-chemical automatic analyzer AU-5200 OLYMPUS (Randox Laboratories, San Francisco, USA). LDL level was determined by the Friedewald formula [9] as follows:
VLDL (mg/dL) = TAG/5
LDL (mg/dL) = TC - VLDL – HDL
Extraction of organs and organ weights
After 6 weeks of test study, animals in all groups were given light anaesthesia using Ethyl Ether in a glass dome and then dissected to extract some organs. Organ weights (liver, heart, kidney, lungs, spleen and testes) were measured and recorded as a percentage of final body weight together with the absolute values while the pancreatic tissues were histologically examined.
Histology of pancreas
Histological examination was based on an earlier protocol [10]. Slices of the pancreatic tissue were fixed in 10% formalin solution for 24 h. All samples were then dehydrated in graded ethanol series, cleared in toluene and embedded in paraffin wax; 5-6 μm sections were routinely stained with Harris hematoxylin and eosins stains (Sigma-Aldrich) and were assessed under light microscope (Nikon Eclipse E400).
Statistical Analysis
Data was analyzed using appropriate statistical methods and program of Microsoft Excel and SPSS v. 19. Results (all mean values) are expressed as group Mean ± SEM (Standard Error of Mean). Comparisons between groups and the significant difference between the control and the experimental groups were analyzed using one way analysis of variance (ANOVA) followed by Duncan’s multiple range tests. P values of < 0.05 were considered statistically significant.
RESULT
Body and Organ Weights
As shown in Table 2 below, the mean body weights of the rats were almost the same (~250 g) in all groups at the onset of the study. At the time of sacrifice, the mean body weight increased significantly (P< 0.05) in groups fed with high-fat diets compared with those groups on normal diets (controls). Percentage increase in mean body weights of rats in DHF and NDHF groups were 41.3% and 40.1% respectively while that of the DNF and NDNF groups (control) were 14.5% and 13.0% respectively. High dietary fat intake had significant impact on body weight. No significant change was observed in the mean weights of organs (liver, heart, kidney, lung, spleen and testes).
Table 2: Effect of composed normal and high-fat diets on body and organ weights of experimental rats (n = 8).
| Experimental Animal Categories | ||||
| DHF | DNF | NDHF | NDNF | |
| Body Weight (g) | ||||
| Initial | 250.00±1.03a | 250.00±1.51a | 250.50±1.67a | 250.00±0.93a |
| Final | 353.17 ±4.32b | 286.33±1.05a | 350.17±3.70b | 282.50±1.34a |
| % increase | 41.3% | 14.5% | 40.1% | 13.0% |
| Organ Weights (g) | ||||
| Lung | 1.17±0.00 | 1.18±0.00 | 1.21±0.07 | 1.20±0.00 |
| Heart | 0.58±0.02 | 0.59±0.00 | 0.57±0.03 | 0.60±0.00 |
| Spleen | 0.73±0.00 | 0.78±0.01 | 0.75±0.02 | 0.77±0.00 |
| Liver | 5.52±0.04 | 5.56±0.00 | 5.50±0.00 | 5.56±0.02 |
| Kidney | 1.44±0.00 | 1.46±0.00 | 1.41±0.03 | 1.43±0.00 |
| Testes | 2.24±0.06 | 2.22±0.01 | 2.25±0.02 | 2.16±0.01 |
Means with the different letter (superscripts) within the same row are significantly different at P value < 0 .05. DHF = Diabetic rats on high fat diet; DNF = Diabetic rats on normal diet; NDHF = Non-diabetic rats on high fat diet; NDNF = Non-diabetic rats on normal diet.
Glycemic Tolerance Test
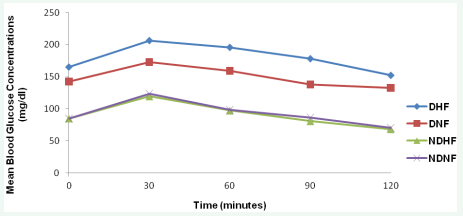
Postprandial glycemic response to high-fat diet showed improved glycemic tolerance over that of normal composed diet. The high-fat fed diabetic rats displayed slower and decreased glycemic response to oral glucose challenge compared with normally-fed healthy and diabetic rats. High-fat fed healthy rats displayed relative normoglycemic response curves. Figure 1 below shows the mean glycemic tolerance curves to test and control diets.
Figure 1: Effect of composed normal and high-fat diets on glycemic tolerance of experimental rats.
DHF = Diabetic grouped rats on high fat diet; DNF = Diabetic grouped rats on normal diet; NDHF = Non-diabetic grouped rats on high fat diet and NDNF = Non-diabetic grouped rats on normal diet.
Lipid Profile
Table 3 below shows the comparative effects of the test diets on the lipid profile of the experimental and control rats. After 6 weeks of diet, levels of the constituting parameters of the lipid profile (except HDL) increased in all groups but much more significant in rats fed with high-fat diets.
Table 3: Effect of dietary high fat intake on lipid profile of experimental rats (n=8).
| Time (weeks) | Experimental groups | |||
| DHF | DNF (Diabetic control) | NDHF | NDNF (Normal control) | |
| Total cholesterol (TC) mg/dl | ||||
| 0 | 52.00±1.47 | 51.20±3.05 | 50.00±3.25 | 51.23±2.25 |
| 6 | 90.50± 1.50b | 59.22±6.85a | 80.05±0.95ab | 57.40 ±2.60a |
| Triacylglycerol (TG) mg/dl | ||||
| 0 | 21.05±1.30 | 20.45±2.00 | 21.00±2.35 | 20.05±2.30 |
| 6 | 72.24±1.00b | 28.34±2.90a | 69.44±1.00b | 29.54±2.70a |
| High density lipoprotein cholesterol (HDL- C) mg/dl | ||||
| 0 | 5.85 ±1.85 | 5.75 ±1.05 | 6.05 ±1.75 | 5.95 ±1.75 |
| 6 | 8.35±2.50a | 10.46±1.34b | 8.00±2.85a | 11.00±1.80b |
| Low density lipoprotein cholesterol (LDL- C) mg/dl | ||||
| 0 | 41.94±7.25 | 41.36±6.30 | 40.00±5.25 | 41.26±6.25 |
| 6 | 67.70± 0.65b | 43.07±1.20a | 58.16±2.00ab | 40.49±0.03a |
Means with the same letter in same row are not significantly different. P value < 0.05 is significant.
DHF = Diabetic rats on high fat diet; DNF = Diabetic rats on normal diet; NDHF = Non-diabetic rats on high fat diet; NDNF = Non-diabetic rats on normal diet
Histological Analysis
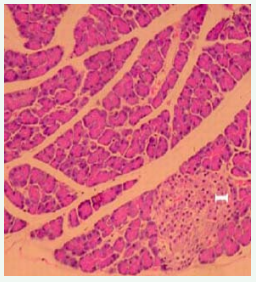
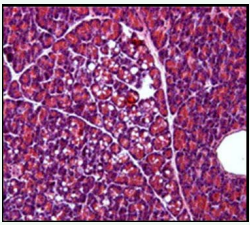
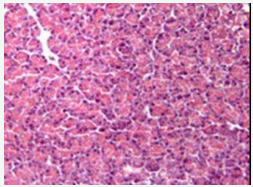
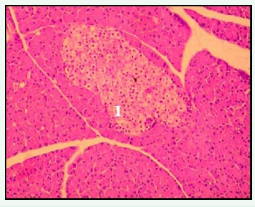
Under high power magnification light microscopic examination, the photomicrographs of the pancreas of the experimental and controlled diabetic and normal rats were closely examined. Pancreatic islet cells from NDNF and NDHF grouped rats exhibited normal histoarchitecture with marked increase in islets size seen in NDNF rats while degenerated islets with vacuolation of β-cells were observed in pancreatic tissue of rats in DHF and DNF Groups (Figures 2-5).
Figure 2: Photomicrograph of the pancreas from normal control (NDNF group) rat demonstrating normohistoarchitecture. Blood capillaries are surrounded by centroacinar cells containing serous acini (hematoxylin and eosin; original magnification x400). I - islet.
Figure 3: Photomicrograph of the pancreas from a diabetic rat on high fat diet (DHF group) showing vacuolation, degranulation and degeneration of serous acini (hematoxylin and eosin; original magnification x400).
Figure 4: Photomicrograph of the pancreas from a diabetic rat on normal feed diet (DNF) showing degenerated serous acini, although some regeneration is also visible (hematoxylin and eosin; origin magnification x400).
Figure 5: Photomicrograph of the pancreas from normal rat on high-fat diet (NDHF group) showing normal pancreatic histoarchitecture with marked increased in islets size (hematoxylin and eosin; original magnification x400). I- Islets.
DISCUSSION
This study determined the effects of high intake of dietary fat on glycemic tolerance (GT), organ weights, lipid profile and pancreatic tissue histology in diabetic rats. The findings obtained from the study revealed increased cardiovascular and metabolic risks associated with consumption of high dietary fat as evidenced by significant weight gain and hyperlipidemia.
Consumption of high fat diet in this study significantly increases the total body weight while the effect on the organ weights was insignificant. At the time of sacrifice (6 weeks post fed), mean body weight of experimental rats was significantly higher (p < 0.05) than control: DHF (353.17±4.32g) NDHF (350.17±3.70g), DNF (286.33±1.05g) and NDNF (282.50±1.34g). This observed increase in the body weight agrees with the findings of few studies [1,12] which suggested that total dietary fat intake is linked to an increased risk of obesity and diabetes.
Glycemic profile (Figure 1) of rats in oral glucose challenge demonstrated a delayed glycemic response and improved glycemic tolerance in diabetic rats fed with high fat diet over that of the diabetic control. The observed pattern of the glycemic profiles agrees with the findings of Pagan [13] in his study which reflects the effect of diabetes mellitus on dietary glucose handling. Presence of large amount of fat in the study diet delays the peak but not the total glucose response as also observed in the studies of Moghaddam [14], Lan-Pidhainy [15] and Boyd [16].
Photomicrographs of the pancreas of the experimental and control rats in this study were closely examined under high power magnification light microscopy. Pancreatic islet cells from NDNF (normal control) rats exhibited normal histoarchitecture, namely clearly visible darkly stained serous acini containing centroacinar cells. Degenerated islets with degranulation and vacuolization of β-cells were observed in pancreatic tissues from diabetic highfat fed rats (DHF group) and diabetic normal-fed rats (DNF groups) as seen in figures 2-5. These observations comply with the findings of other study using alloxan-induced diabetic animal models [17]. Regeneration of some islets cells observed in the photomicrograph of the pancreas in the DNF grouped rats raises some hope of improving the course of diabetes with good dietary recommendations.
Analysis of the lipid profile showed significant increase in total cholesterol, triglycerides and low density lipoprotein levels in high-fat fed experimental healthy and diabetic rats as shown in Table 3 above. This observed hyperlipidemia demonstrates the impact of high fat diets on blood lipid chemistry as supported by results of other studies [18,19]. This abnormal increase in the levels of lipid components calls for reasonable caution. However, moderate consumptions of fatty foods according to some epidemiological studies [20-22] have revealed reasonable reductions in metabolic and cardiovascular risks associated with obesity.
CONCLUSION
High intake of dietary fat caused significant increase in total body weight, altered ultrastructure of pancreatic tissue and hyperlipidaemia in diabetic rats with delayed peak glycemic response and improved glycemic tolerance. While achieving good glycemic tolerant effect of fat in diets, recommendation of dietary fats in diabetic menu should be rationalized to minimize cardiovascular and metabolic risks associated with high-fat diets.
ACKNOWLEDGEMENT
We acknowledge Mr. Alabi of Animal Science and Nutrition Department who prepared the study diets.
REFERENCES
1. Astrup A. Dietary management of obesity. JPEN J Parenter Enteral Nutr. 2008; 32: 575-577.
10. Humason GP. Animal tissue techniques. San Fransisco: W.H. Freeman and Company; 1979
11. Astrup A1. Dietary management of obesity. JPEN J Parenter Enteral Nutr. 2008; 32: 575-577.
13. Pagan, JD, Geor RJ, Caddel SE, Pryor PB, Hoekstra KE. The relationship between glycemic response and the incidence of OCD in Thoroughbred weanlings: A field study. In: Proc. 47th AAEP Conv, 2001; 322-325.