Production and Quality Evaluation of Wine from Watermelon Juice and Ginger Extract
- 1. Department of Food Science and Technology, University of Agriculture, Nigeria
- 2. Department of Chemistry, Benue state University, Nigeria
- 3. Department of Agriculture Engineering, Akperan OrshiiCollege of Agriculture, Nigeria
Abstract
Post-harvest loss is a very serious problem in developing countries which leads to low income earning of the farmers and food shortage. This is due to the insufficient storage facilities as well as the technical know-how of post-harvest handling from the harvesting period to storage and also lack of proper marketing channel. The aim of this research was to control postharvest loses in watermelon and ginger and also to develop health promoting and acceptable wine using watermelon (Citrullus lanatus) and Ginger (Zingiber officinale). The wine was produced using ratios of 100:00, 95:05, 90:10, 85:15, 80:20, 75:25 watermelon juice: ginger extract designated as sample A-F respectively. The sample from Watermelon juice alone served as the control. Fermentation of the wine was carried out in two phases the primary and secondary phase. During the fermentation process, the pH, specific gravity (SPG) and alcoholic content was monitored on a daily basis. The results of physico-chemical analysis revealed that titrable acidity ranged from 0.04 to 0.10% while pH from 4.76-4.68, total soluble solids (brix level) ranged between 3.5-4.0 0 brix, and specific gravity from 1.01-1.04. Vitamin C contents ranged from 1.95-2.85 mg/100g. The alcoholic content of the wine ranged from 2.30-8.50% with sample A (the control) having the highest alcoholic content. Antioxidant assays shows that samples with highest level of ginger extract show stronger antioxidants in general before and after fermentation. The antioxidant assays shows that fermentation improves the antioxidant properties of watermelon juice- ginger extract blends. The results for minerals shows a range 2.79-3.79mg/L,0.06-0.29mg/L, 7.60-27.37mg/L and 0.24-0.66mg/L for Ca, Cu, Fe and Zn respectively. The Zn and Cu contents increased in the wine after fermentation while Fe and Ca decreased. The results shows that the mineral content of the wine increase with increase in ginger. Findings of microbial studies showed the formulated wine is safe for human consumption. Sensory evaluation shows that sample B having the least ginger (5%) was rated highest in colour, taste and general acceptability. The colour ratings decrease from 5.30-5.0 as the level of ginger increases from 10-25% level. The taste ratings also decrease from 4.90 to 4.30 as the level of ginger increases from 10-25% level. The flavor ratings increase as the level of ginger increases in the wine. This research indicates that an acceptable, enhanced physico-chemical and mineral with improved antioxidant properties wine could be produced from blends of watermelon juice and hot water ginger extract.
Keywords
• Post harvest losses
• Watermelon juice
• Ginger extract
• Wine
• Antioxidant properties
• Acceptability
Citation
John PG, Yusufu MI, Ahemen SA (2018) Production and Quality Evaluation of Wine from Watermelon Juice and Ginger Extract. J Hum Nutr Food Sci 6(1): 1122
INTRODUCTION
Wine is a product of alcoholic fermentation by yeast of the juice of ripe grapes or any fruit with a good proportion of sugar and one of the most recognizable high value added products from fruits [1,2]. The production of wine from fruit juice is a form of food preservation since fruits are highly perishable. Most commercially produced wines are usually made from fermented grapes [3]. Wine could be produced from watermelon juice blended with ginger extracts.
Watermelon (Citrullus lanatus) which is grown in both tropical and subtropical regions have a lot of nutritional and health benefits. It is known to be rich in electrolytes and water content; low in calories and fats and yet a very rich source of numerous health promoting phyto-nutrients and antioxidants that are essential for optimum health. Ginger; belongs to Zingiberaceae family [4,5]. Ginger can be available in different commercial products like cookies; candy; teas; tinctures; sodas; jam; beer; capsule and syrup [6]. The chief active constituents of ginger are Volatile oil (zingiberene; zingiberol; D-camphor); Shogaols; Diarylheptanoids; Gingerols; Paradol; Zerumbone; 1-Dehydro-(10) gingerdione; Terpenoids and Ginger flavonoids [7]. Shogaols and Gingerols are responsible for ginger’s pungency [8]. Ginger has a wide range of biological activities that are attributed to its active constituents [9]. Ginger is one of the most important plants with several medicinal; nutritional and ethno medical values therefore; used extensively worldwide as a spice; flavorings agent; herbal remedy and antioxidant properties.
Post-harvest loss is a very serious problem in developing countries which leads to low income earning of the farmers and food shortage. This is due to the insufficient storage facilities as well as the technical know-how of post-harvest handling from the harvesting period to storage and also lack of proper marketing channel.
The utilization of water melon and ginger in wine production could be advantageous and will go a long way in enhancing their utilization.
Therefore; in view of the above benefits of watermelon and ginger; conversion into a value added product like wine will be very useful. The objective of this research therefore was to produce and evaluate the quality of wine from blends of watermelon juice and ginger extract with the view of controlling the post-harvest losses of these valuable plant foods.
MATERIALS AND METHODS
Materials
The water melon and ginger used for this research work were bought from modern market; Benue State; Nigeria.
Methods
The watermelon fruit and ginger root were thoroughly sorted and graded to remove bad ones from the lot. The sorted fruits and ginger root was then washed to remove adhering soils; dirt and extraneous materials. The watermelon was peeled and then the seeds were removed. It was then sliced into smaller pieces for easy blending using a blender for juice extraction. 750g of the ginger was extracted with 1000 ml of boiled water at 100°C with stirring at 150 rpm for 3 h with 1000ml de ionized water to obtain water extract. It was cooled to room temperature and filtered. The residues were then extracted two with 1000 ml additional boiled water at 100°C as described above. The combined extract was evaporated at 60°C in a rotary evaporator to a viscosity of 4 centipoises [10].
It was then cooled again at room temperature and then blended with the watermelon juice in various proportions. Sugar and yeast were added and left to ferment for 5 days for primary fermentation followed by secondary fermentation for another 5 days. It was then clarified; preserved using potassium meta bisulphite; pasteurized; cool; bottled; labeled; sealed and allowed to age for one month [2]. The wine was produced using ratios of 100:00; 95:05; 90:10; 85:15; 80:20; 75:25 watermelon juice: ginger extract designated as sample A-F respectively.
Physico- chemical analysis
The pH; Total solids; Ascorbic acid; TTA as% citric acid was determined as according to standard methods [11,12].
The pH was then determined using fisher science Education pH meter (model 90526 Singapore) by inserting the pH probe into the slurry. The readings were noted from the range 0-14 of the pH meter
For TTA; Twenty milliliters of each sample was collected in a beaker; one 1ml of phenolphthalein indicator was added and was titrated with 0.1m NaOH to pink colour. The total titratable acidity was calculated as percentage citric acid as shown by the equation
% TTA = volume at base × normality of base × millimeter equivalent × 100
Ascorbic acid (vitamin C) was determined using titrimetric method (2; 2; 6 dichlorophenol indophenol as a dye).
The total solids content was expressed as a ratio of weights obtained before and after the drying process. One hundred (100 mL) of the sample was placed in an oven at a temperature of 105°C until a steady mass is obtained. Total soluble solids were determined using the Abbé refractometer (PZO-RL1; Warszawa; Poland).
Microbiological analysis
The total bacteria; fungi; yeast and coliform count were determined. For fungi count; the media was treated with tartaric acid to inhibit the growth of bacteria. Enumeration of microorganisms was by serial dilution techniques. Petri-dishes were sterilized in an autoclave at 121°C for 15min. Samples were homogenized and was transferred aseptically into the test tube using 1 ml sterile pipette and tenfold serial dilution was carried out. The plates were incubated at 37°C for 24hrs. The colonies were counted per plate using colony counter and expressed in colony forming unit per ml (CFU/mL) and values were estimated by means of triplicate determination [13].
Mineral analyses
The determination of Ca; Cu Fe and Zn was carried out as described by AOAC [11]. The samples were digested with a mixture of nitric acid and perchloric acid in the ratio of 10:4 (v/v) on hot plates sand bath. After complete digestion; samples were cooled to room temperature and appropriately diluted and were analyzed by Atomic Absorption Spectrometry.
Antioxidant properties
Determination of DPPH radical scavenging activity (DRSA); Ferric reducing antioxidant power (FRAP); Metal chelating activity (MCA) and Hydroxyl radical scavenging activity (HRSA) were carried out as described by [14].
Sensory evaluation
Sensory evaluation of the watermelon- ginger wine samples was carried out by 20 trained panelists. Sensory attributes evaluated were taste; aroma; colour; flavor and overall acceptability using seven point hedonic scale of 1 to 7 were I indicates extremely disliked; 2 indicates very much disliked; 3 indicates moderately disliked; 4 indicates neither liked nor disliked; 5 indicates liked moderately; 6 indicates very much liked and 7 indicate extremely liked [15].
Statistical analysis
The data was analyzed using a statistical software package SPSS design 8.07 (stat Inc. Minneapolis; USA) and was used to generate the experimental design matrix and analyze the experimental data [16].
RESULTS AND DISCUSSIONS
Physico-chemical properties of the water melon ginger extract
Table 1 shows the physico-chemical properties of watermelon juice and the ginger extract.
|
Table 1: Physico-chemical properties of blends of watermelon juice and ginger extract. |
||||||
|
Samples |
pH |
°BRIX |
SPG |
VITC(mg/100g) |
TTA % |
TS % |
|
A |
5.12f ± 0.01 |
8.00a ± 0.00 |
1.04a ± 0.00 |
3.61a ± 0.40 |
0.14a ± 0.00 |
2.99d ± 0.00 |
|
B |
5.18e ± 0.01 |
8.00a ± 0.00 |
1.02a ± 0.10 |
2.60c ± 0.17 |
0.13b ± 0.01 |
2.95c ± 0.01 |
|
C |
5.37d ± 0.00 |
8.00a ± 0.00 |
1.01a ± 0.30 |
2.52b ± 0.16 |
0.11c ± 0.01 |
2.91b ± 0.01 |
|
D |
5.41c ± 0.01 |
7.97a ± 0.06 |
1.01a ± 0.60 |
2.49d ± 0.08 |
0.10d ± 0.01 |
2.80b ± 0.02 |
|
E |
5.46b ± 0.00 |
7.67a ± 0.58 |
1.02a ± 0.40 |
2.60bc ± 0.04 |
0.10d ± 0.01 |
2.77a ± 0.01 |
|
F |
5.49a ± 0.01 |
7.00a ± 0.00 |
1.02a ± 0.30 |
2.40e ± 0.20 |
0.09e ± 0.01 |
2.71b ± 0.02 |
|
Means are ± SD of triplicate determinations and values bearing different superscript in a column are significantly different (P≤0.05) from each other. SPG- Specific gravity, VIT C- Vitamin C, TTA- Titrable acidity, TS- Total solid Blend formulation Samples: A B C D E F WG 100:00(control) 95:05 90:10 85:15 80:20 75:25 |
||||||
Before fermentation; the pH values ranged from 5.12-5.49. There were significant differences among the samples. Sample F with the highest level of ginger had significantly higher pH value. The result indicates that watermelon juice is more acidic than the ginger extract.
The total soluble solids (brix) for the samples ranged from 7.00- 8.00. There was no significant difference among the samples with respect to total soluble solids. The specific gravity for the samples ranged from 1.01-1.04 and there was no significance difference amongst samples. The vitamin C ranged from 2.40-2.61 mg/100g. There were significant differences among the samples. The values decrease with increase in ginger in the blends. The decrease in values may be attributed to dilution effect of ginger. Vitamin C is reported to be high in watermelon juice than ginger.
The total titrable acidity (TTA) ranged from 0.09-0.14%. There were significant differences among the samples. The inverse relation between the pH and the TTA indicates that pH increase lead s to reduction in acidity of watermelon juice ginger extract blends. This is in agreement with the studies [17]. The total solids ranged from 2.71-2.99%. There was a significant difference among the samples. The values decrease as the level of ginger increases. The decrease could be attributed to dilution effect. The ginger extracts appear to be lower in total solids than the watermelon juice
Physico-chemical properties of the wine samples
Table 2 shows the results of physico-chemical properties of the wine after fermentation.
|
Table 2: Physico-chemical properties of the wine produced from watermelon juice and ginger extract. |
||||||
|
Samples |
pH |
°BRIX |
SPG |
VITC(mg/100g) |
TTA % |
TS % |
|
A |
4.68e ± 0.01 |
4.00a ± 0.00 |
0.99e ± 0.00 |
2.85a ± 0.19 |
0.10a ± 0.00 |
1.58d ± 0.02 |
|
B |
4.68e ± 0.00 |
4.00a ± 0.36 |
1.00d ± 0.01 |
2.49b ± 0.08 |
0.09b ± 0.01 |
1.71a ± 0.01 |
|
C |
4.69d ± 0.00 |
3.93b ± 0.35 |
1.00c ± 0.00 |
2.54b ± 0.28 |
0.08c ± 0.00 |
1.65b ± 0.01 |
|
D |
4.71c ± 0.01 |
3.83c ± 0.76 |
1.01b ± 0.00 |
2.35bc ± 0.05 |
0.06d ± 0.02 |
1.73a ± 0.02 |
|
E |
4.74b ± 0.00 |
3.77d ± 0.12 |
1.00c ± 0.00 |
2.17cd ± 0.11 |
0.05e ± 0.00 |
1.64b ± 0.02 |
|
F |
4.76a ± 0.00 |
3.55e ± 0.93 |
1.04a ± 0.00 |
1.95d ± 0.14 |
0.04f ± 0.00 |
1.61c ± 0.01 |
|
Means are ± SD of triplicate determinations and values bearing different superscript in a column are significantly different (P≤0.05) from each other. SPG- Specific gravity, VIT C- Vitamin C, TTA- Titrable acidity, TS- Total solids Blend formulation Samples: A B C D E F WG: 100:00(control) 95:05 90:10 85:15 80:20 75:25 |
||||||
It was observed that the pH decreases after the fermentation. The pH increases with the level of ginger with no significance difference and the least is sample A which is the control. The value for the pH ranges from 4.76- 4.68 from sample F to A. The variation of the pH may be due to the concentration of the ginger in various formulations and as a result of fermentation. The decrease in the pH values after fermentation could be attributed to the production of acids in the fermenting medium.
Studies have shown that during fermentation of fruits; low pH is inhibitory to the growth of spoilage organisms but create conducive environment for the growth of desirable organisms. Also; low pH and high acidity are known to give fermentation yeast comparative advantage in natural environments and maximum pH for mixed fruit wine was reported to be 3.5 [17,18] . A similar observation has been reported by Reddy and Reddy in their study on mango fruit; optimum pH and temperature values for quality wine production was 5.0 and 30°C; respectively.
Acidity plays a vital role in determining wine quality by aiding the fermentation process and enhancing the overall characteristics and balance of the wine. Lack of acidity will mean a poor fermentation [19]. The pH is slightly acidic; this confers stability on the wine sample and the specific gravity ranged from 0.784 to 1.020 [20]. There exists a correlation between PH and acidity of the sample. The higher the acidity; the lower the pH of the wine. A similar study conducted by [21] revealed that there is a corresponding reduction in pH as the acidity increased in sour-sop juice. The titratable acidity decrease from sample A to F. After fermentation; the sugar (°brix) level decrease with sample A having the highest value of 0.10 and F the least 0.5. The °brix was higher in samples A and B and the values decreases as the level of ginger increases. Specific gravity (SPG) was observed to be higher in sample F and the least was 0.99 for sample A which is the control.
The specific gravity and sugar (o Brix) of each sample decreased after fermentation. This could be due to microbial succession; available nutrients; sugar and alcohol resulting in the production of acid. These results are in agreement with the report by Querol et al. [22].
Vitamin C content of the wine decrease from sample A- F with A having the highest value of 2.85 mg/100g and F 1.95 mg/100g. The decrease in vitamin C content may be due to the dilution effect of ginger. The ascorbic acid acts as an antioxidant to help prevent molecular changes caused by oxidation and as a promoter of iron absorption [23].
Antioxidants activity of watermelon juice-ginger extracts blends and the wines
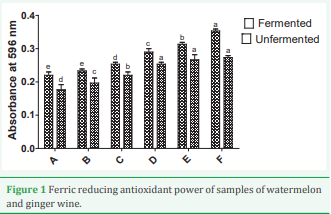
Figure 1 shows ferric reducing antioxidant power activity (FRAP) of watermelon juice-ginger extracts blends and the resultant wine. The FRAP increases as the level of ginger increased for watermelon juice-ginger extract and the resultant wines.
Figure 1: Ferric reducing antioxidant power of samples of watermelon and ginger wine.
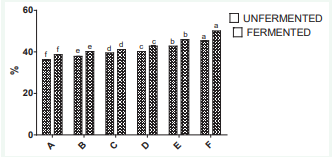
The metal chelating power activity is shown in Figure 2.
Figure 2: Metal chelating activity of samples of wine produced from watermelon and ginger
The activity also shows an increase after fermentation for all samples with increase in ginger.
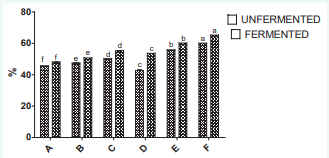
Figure 3 shows the hydroxyl radical activity (HRSA) of the samples for both fermented and unfermented which also shows and increases of the activity with the increase in ginger level.
Figure 3: Hydroxyl radical scavenging activity of samples of wine produced from watermelon and ginger.
The fermented samples (wine) shows higher activity than the watermelon juice-ginger extract blends
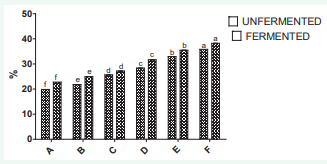
The diphensyl-1-pierylhydrazyl scavenging activity is shown in Figure 4.
Figure 4: DPPH radical scavenging activity of samples of wine produced from watermelon and ginger extract. Minerals of the watermelon juice and ginger extract blends.
The results also show an increase in the scavenging activity for the fermented and unfermented samples as the level of ginger increases. The wine samples also shows higher DPPH activity than the corresponding blends from watermelon juiceginger extract
All the figures shows that fermentation improves the antioxidant properties of watermelon juice ginger extract blends.
The watermelon-ginger blends had lower antioxidant activities than the resultant wine samples in sequential order with sample A having the least value while F have the highest value respectively with no alcoholic content. The antioxidant activities differ significantly in the produced wine. The magnitude of the difference depends on the assay employed. This result is well in accordance with recent reports in the literature suggesting a positive correlation between phenolic compound contents and the antioxidant capacity [24]. Phytochemical investigation of several types of ginger rhizomes has indicated the presence of phenolic compounds [25].
The FRAP shows an increase in the scavenging activities from sample A to F respectively. The highest is sample F with the value 0.35 followed by sample E which have a significance difference with C and D of (P≤00.05). The least is sample B and A which show no significance difference. The reducing power property indicates that the antioxidant compounds are electron donors and can reduce the oxidized intermediates of the lipid peroxidation process.
The wine had a moderate increase in the inhibitory in linoleic acid oxidation from 0.50 to 1.22 which may be explained by the possible complex constitution of the phenolic compounds in the different fractions of the blend formulations of the wine. The result that the wine is effective in protecting linoleic acid against oxidation and might be beneficial for health against diseases related to oxidation [26].
The metal chelating power of the wine increased from 36.63 to 50.07% which is significantly high. The chelating ability increased with the increase of the ginger in the watermelon juice. In this study; the results indicate that the watermelon ginger extract wine has many active components for chelating metal ions. The metal chelating ability may be attributed to the presence of total phenols and flavonoids.
For the OHRS; all the samples generally were found to have a certain capacity to scavenged the hydroxyl radical; and sample F with the highest ginger extract in the blend formulation of the wine with the value 65.31.The OH capacity of the wine appears to highly correlate with the inhibition of lipid peroxidation; which might be attributed to the combined effects of reducing power; hydrogen atom donation and active oxygen scavenging. These results suggest that the antioxidant activity of the watermelon ginger wine may not be exclusive due to the specific bioactive compounds.
The values for the DPPH scavenging of the wine ranged from 22.7 to 38.23 from sample A to F respectively. The bleaching activity is mainly from the presence of polyphenols and flavonoids; especially phenolic with a substitution of hydroxyl groups in the aromatic rings; thus easily demonstrating the hydrogen-donating capacity [27]. The watermelon ginger wine exerted a concentration-dependent manner on the DPPH scavenging activity which might indicate that there are some compounds being contributors on scavenging the DPPH radical. It was reported that more than 50 compounds have been identified including gingerols or diarylheptanoids from ginger with antioxidant activity [28].
Table 3 shows the values of minerals present in the produced watermelon juice and ginger extract.
|
Table 3: Selected minerals composition of watermelon juice-ginger extract samples (mg/100g). |
||||
|
samples |
Ca(mg/100g) |
Cu(mg/100g) |
Fe(mg/100g |
Zn(mg/100g) |
|
A |
3.70e ± 0.00 |
0.01f ± 0.00 |
0.15e ± 0.01 |
0.11f ± 0.01 |
|
B |
3.76d ± 0.01 |
0.03e ± 0.01 |
0.21d ± 0.01 |
0.13e ± 0.01 |
|
C |
3.77d ± 0.06 |
0.06d ± 0.00 |
0.49c ± 0.00 |
0.16d ± 0.01 |
|
D |
3.80c ± 0.00 |
0.16c ± 0.01 |
0.53b ± 0.00 |
0.19c ± 0.01 |
|
E |
4.67b ± 0.00 |
0.26b ± 0.00 |
1.07a ± 0.06 |
1.07a ± 0.06 |
|
F |
4.87a ± 0.03 |
0.29a ± 0.00 |
1.07a ± 0.06 |
1.10a ± 0.00 |
|
Means are ± SD of triplicate determinations and values bearing different superscript in a column are significantly different (P ≤ 0.05) from each other. |
||||
Some of the minerals present in the fruit wines produced are required for metabolic functioning of the body. Minerals are important for bone and teeth formation; blood clotting; muscle contraction; transmission of impulses in nerves and maintenance of osmotic balances.
Watermelon and ginger contain significant amount of minerals of which can be found in the produced wine [29]. There was significant increase in minerals of the samples with increase in ginger. The highest in Ca was sample F with the highest ginger blends with the value 4.87mg/100g and the least is sample A with values 3.70mg/100g which is the control 100% watermelon. The Zn values ranged from 0.17 mg/100g for sample C and the least is with 0.11mg/100g. Copper was highest in sample F with values 0.29mg/100g followed by sample A with values 0.01mg/100g. For the Fe; sample F and E have the highest values of 1.07mg/100g with no significance difference and the least is sample A which is the control with the value 0.15mg/100g.
Minerals composition of the wine samples from blends of water melon and Ginger extract
Table 4 shows the mineral content of wine.
|
Table 4 Selected minerals composition of wine samples from blends of water melon-ginger extract blends (mg/100g): |
||||
|
Samples |
Ca(mg/100g) |
Cu(mg/100g) |
Fe(mg/100g) |
Zn(mg/100g) |
|
A |
2.79e ± 0.01 |
0.06e ± 0.00 |
7.60f ± 0.00 |
0.24e ± 0.00 |
|
B |
2.87d ± 0.00 |
0.12d ± 0.02 |
10.87e ± 0.06 |
0.52d ± 0.02 |
|
C |
2.87d ± 0.00 |
0.20c ± 0.01 |
14.33d ± 0.12 |
0.56c ± 0.02 |
|
D |
3.19c ± 0.01 |
0.23b ± 0.00 |
18.53c ± 0.12 |
0.60b ± 0.00 |
|
E |
3.38b ± 0.01 |
0.24b ± 0.01 |
24.43b ± 0.12 |
0.61b ± 0.02 |
|
F |
3.87a ± 0.00 |
0.29a ± 0.00 |
27.37a ± 0.06 |
0.66a ± 0.01 |
|
Values are means of triplicate determinations. Means within a column with different superscript are significantly different. |
||||
This result shows that fermentation decreases the mineral concentration of the wine in Ca and Fe with increase in ginger. The decrease in the level of Ca and Fe in the wine may be attributed to the utilization of these minerals by the fermenting organism. The Cu and Zn were observed to increase slightly after fermentation. The highest with Ca content is sample F with the value 3.87mg/100g with 25%of ginger and the least is sample A which is the control with 100% watermelon. There were significant a difference amongst samples B; C and E. Zn was found to be highest in sample F with values 1.66mg/100g followed by sample E with the values 1.61mg/100g while sample D and E have no significance difference and cupper increases after fermentation. The highest is sample F with 0.34mg/100g and the least is sample A with 0.06mg/100g. Fermentation is reported to increase the availability of minerals through hydrolysis [30].
Microbial load of water melon Ginger extract blends
The values for the microbial population are shown in Table 5.
|
Table 5: Microbial load of blends of watermelon juice and ginger extract. |
|||
|
Samples |
TBC (×105CFU/ml) |
TCC (×102CFU/ml) |
TFC (×105CFU/ml |
|
A |
1.10f ± 0.10 |
0.00 ± 0.00 |
4.37a ± 0.12 |
|
B |
2.72e ± 0.03 |
0.00 ± 0.00 |
3.90b ± 0.10 |
|
C |
3.10d ± 0.12 |
0.00 ± 0.00 |
3.63c ± 0.12 |
|
D |
3.17c ± 0.06 |
0.00 ± 0.00 |
3.43d ± 0.15 |
|
E |
3.37b ± 0.12 |
0.00 ± 0.00 |
3.13e ± 0.06 |
|
F |
3.83a ± 0.06 |
0.00 ± 0.00 |
3.03f ± 0.06 |
|
Means are ± SD of triplicate determinations and values bearing different superscript in a column are significantly different (P≤0.05) from each other. Blend formulation Samples: A B C D E F WG: 100:00(control) 95:05 90:10 85:15 80:20 75:25 Key: TVC- Total bacteria count, TCC- Total coliform count, TFC- Total fungal count Microbial load of the wine samples |
|||
The microbial load ranges from 7.20 x 104 CFU ml-1 - 3.43 x 105 CFU ml-1 and 6.30 x 105 CFU ml-1 for total bacteria and total fungi respectively. There was no coliform growth at the dilution factors used.
The results of microbial enumeration of the wine samples are shown in Table 6.
|
Table 6: Microbial load of wine samples from blends of watermelon juice and ginger extract. |
|||
|
Samples |
TBC (×103CFU/ml) |
TYC (×103CFU/ml) |
TFC (×102CFU/ml) |
|
A |
1.23a ± 0.15 |
0.83e ± 0.15 |
1.67c ± 0.58 |
|
B |
2.33cd ± 1.15 |
2.67ab ± 1.15 |
1.33c ± 0.58 |
|
C |
5.33a ± 0.58 |
3.07a ± 0.21 |
6.67a ± 1.15 |
|
D |
4.67ab ± 1.15 |
0.43e ± 0.15 |
5.67a ± 0.58 |
|
E |
3.67bc ± 1.15 |
1.63cd ± 0.25 |
3.67b ± 0.58 |
|
F |
1.67c ± 0.58 |
2.03bc ± 0.25 |
1.33c ± 0.58 |
|
Means are ± SD of triplicate determinations and values bearing different superscript in a column are significantly different (P≤0.05) from each other. Blend formulation Samples: A B C D E F WG: 100:00(control) 95:05 90:10 85:15 80:20 75:25 Key: TVC- Total bacteria count, TYC- Total yeast count, TFC- total fungal count |
|||
The values ranges from 1.23 x 103 CFU ml-1 – 5.33 x 103 CFU ml-1; 1.33 x 102 cfu ml-1 – 6.67 x 102 CFU/ml respectively for total bacteria; total coliform and total fungi. This implies that the beverage was produced under hygienic conditions and is safe for human consumption [31]. Therefore; the total viable count was within the acceptable limit according to Sri Lanka standard for all drinks / beverages and juices [32]. Many products that could safely be maintained sterile by a pasteurization process alone could be doubly preserved by the addition of potassium metabisulphites. The sulphites inhibit yeast; moulds and bacteria [33].
Sensory attributes of the wine samples from blends of watermelon-ginger extract
The results of sensory evaluation of the samples of wine from watermelon and ginger extract are shown in Table 7.
|
Table 7: Mean sensory scores of samples of wine produced from watermelon juice and ginger extract (mg/100g). |
||||
|
Samples |
Colour |
Taste |
Flavour |
Overall acceptability |
|
A |
5.30b ± 1.07 |
5.20ab ± 0.92 |
4.80d ± 0.92 |
4.90e ± 1.10 |
|
B |
5.40a ± 0.84 |
5.60a ± 0.84 |
4.40a ± 1.25 |
5.40a ± 0.82 |
|
C |
5.30b ± 0.97 |
4.90c ± 0.88 |
5.20d ± 1.03 |
5.30b ± 0.95 |
|
D |
5.10c ± 0.74 |
4.90c ± 1.10 |
5.10bc ± 0.88 |
5.30b ± 0.67 |
|
E |
5.00d ± 0.67 |
4.90c ± 0.88 |
5.30bc ± 0.82 |
5.20c ± 0.92 |
|
F |
5.00d ± 0.67 |
4.30ab ± 0.95 |
5.40a ± 1.07 |
5.00d ± 0.82 |
|
Means are ± SD of triplicate determinations and values bearing different superscript in a column are significantly different (P≤0.05) from each other. Blend formulation Samples: A B C D E F WG: 100:00(control) 95:05 90:10 85:15 80:20 75:25. |
||||
The results shows that sample B having the least ginger (5%) was rated highest in colour; taste and general acceptability. The colour ratings decrease from 5.30-5.0 as the level of ginger increases from 10-25% level. The taste ratings also decrease from 4.90 to 4.30 as the level of ginger increases from 10-25% level. The flavor ratings increase as the level of ginger increases in the wine. There were significant differences among the wine samples with respect to flavor. The overall acceptability ratings decrease gradually as the level of ginger increases. The wine samples were rated high generally thus can be prepared for commercial purpose to serve as a special wine with similar constituents as other already existing commercial wines [34].
CONCLUSION
This study has demonstrated that wine of good quality could be produce from watermelon juice and ginger extract. The antioxidant properties determined by DPPH; MCA; FRAP; LAO and HRSA has also demonstrated improved antioxidant properties in the watermelon juice and ginger extract wine.
REFERENCES
1. Okafor N. “The technology of passion fruit and Pawpaw wines”. Am J Enol Vitic. 2007; 17: 27.
2. Jacobs F. Making wine from pineapple. Ihem Davis Press. Owerri. 2001.
5. Ravindran P, Babu KN. Ginger: The genus zingiber CRC Press. 2004.
6. Maxwell I. Let’s make ginger beer. Dave’s Garden. 2008.
11. AOAC Official Methods of Analysis (22nd Edn). Association of Official Analytical Chemists. Washington USA, 2005.
12. Harrigan WF. Laboratory methods in Microbiology Academics Press, Califonia, USA, 1998.
13. Musa KH, Abdullah A, Jusoh K, Subramaniam V. Food Analysis Methods. 2010; 4: 100-107.
15. Larmond E. Laboratory method of sensory evaluation of food (PublNo1637). Dept of Agriculture Ottawa, Canada. 1979.
19. Berry CJJ. First steps in wine making. published by G.W. Kent, inc. 3667 morgan road, Ann Arbor M I 48108, 2000; 235.
23. Wardlaw GM. Perspectives in Nutrition, McGraw-Hill. USA. 1999; 443.
27. Anononymos. Making Wine at Home Using Wild Yeast. Making Wine from Wild Yeast. 2008.
32. Frezier CW, Westhoff CD. Food Microbiology. 4th Edn. New Delhi. Tata McGraw-Hill. 1995; 339-345.
33. Ghana Standard Board GSB. Specification for Fruit Squashes and Fruit Cordials; 1995; 168.
34. Carter HW, Charley VLS, Bristol C. The preservation of fruit juice products with special reference to nutritional value. J Cambridge. 2007; 8: 12-14.