Relationship between Different Iodine Status during Pregnancy and Infantile Physical Development in China
- 1. Department of Nutrition and Food Hygiene, Tianjin Medical University, China
- 2. Department of Epidemiology and Biostatistics, Guangdong Medical University, China
- 3. Department of Endocrinology and Metabolism, Tianjin Medical University General Hospital, China
INTRODUCTION
Iodine is an essential microelement to maintain human health [1, 2]. Especially, during the first 1,000 days of life, adequate iodine intake is unequivocally important for infantile physical and brain development [3]. The iodine requirement increases during pregnancy due to the transfer of iodine to the fetus and increased maternal thyroid hormone synthesis [4]. Therefore, pregnant women are highly vulnerable to iodine deficiency.
Approximately 90% of the iodine ingested by the body is excreted in the urine, so urine iodine is a good indicator of recent iodine intake. Median urinary iodine excretion is a recommended biomarker for monitoring daily iodine intake in specific populations [5,6]. World Health Organization (WHO)/ United Nations International Children’s Emergency Fund (UNICEF)/ International Council for Control of Iodine Deficiency Disorders (ICCIDD) recommended an adequate median urine iodine concentration (UIC) of 150 249μg/L for pregnant women, higher than that for reproductive women (100-199μg/L) [7]. The latest national iodine deficiency disease surveillance reported that the iodine status of residents in China was appropriate. But in six provinces and cities, the median UIC for pregnant women was lower than 150μg/L, indicating mild iodine deficiency [8].
Iodine deficiency during lactation will affect the iodine nutritional status and thyroid function of infants, and even negatively affect their growth and development [9]. But, evidence on the effects of iodine status during pregnancy on infantile growth and development is limited. Severe iodine deficiency during pregnancy can lead to a series of adverse pregnancy outcomes, including thyroid dysfunction, miscarriage, and stillbirth, even congenital cretinism and growth retardation in infants [3,10]. However, current research on the relationship between mild iodine deficiency in pregnancy and physical development is inconclusive. Some studies reported adverse effects of mild iodine deficiency during pregnancy on infantile growth and development [11,12]. However, results from other cohort studies have shown no differences in infantile physical development between mothers with mild iodine deficiency [9,13].
Due to the success of salt iodization programmer commonly implemented in China, public health attention has shifted to mildto-moderate iodine deficiency, which is still prevalent in some areas, especially among pregnant women. Currently, there are few and controversial studies on the relationship between iodine status during pregnancy and physical development. This study was aimed to investigate physical development of infants at 18- 24 months old under different maternal iodine status during pregnancy and evaluate the relationship between iodine status during pregnancy and physical development of infants in China. This prospective study compared different maternal iodine levels in relation to infant physical development.
MATERIALS AND METHODS
Subjects and Design
This study is a part of an ongoing prospective study conducted in Tianjin and Wuqiang, which was designed to explore the relationship between iodine status during pregnancy and neonatal Thyroid stimulating hormone (TSH) and infants’ physical growth at 18-24 months old. These two areas are adjacent, with similar geography, culture, dietary habits, and food and water iodine concentration. Wuqiang is a county in Hebei Province, where the iodine content in drinking water is <15.0μg/L and both crude salt (non-iodized) and refined iodized salt are consumed. In a pilot study among 77 pregnant women in Wuqiang the 24-h UIC was 108 (69, 163) μg/L, and 67.6% of the UIC values were <150μg/L. Therefore, ?Wuqiang was chosen as a mildly iodine-deficient region for this study. Tianjin, where iodized salt is the main source of iodine, is iodine sufficient (8).
All subjects were recruited from the Departments of Obstetrics in ?Tanggu Maternity Hospital, Tianjin and the Wuqiang Center of Diseases Prevention and Control, Hebei from March 2016 to June 2018. All pregnant women presenting for their routine antenatal care during pregnancy were invited to participate in our study after a general description of the project. The gestational week was determined based on the results of the ultrasound examination of the pregnant woman’s abdomen or the time of the last menstruation. Women aged 20-35 years with no previous history of thyroid disease, no use of thyroidrelated medications, and no other chronic diseases were eligible for inclusion. Pregnant women who had lived in the local area for less than 5 years or who were using iodine supplements were excluded. Pregnant women were recalled back 18-24 months after delivery and were investigated, until to December 2019, 469 mother-infant pairs were recruited. All research protocols were approved by the Medical Ethics Committee of Tianjin Medical University, all procedures performed in the study were in accordance with the ethical standards of the committee and with the 1964 Helsinki declaration. All participants provided written informed consent after research protocols were carefully explained to them (Ethical approval number: NCT03710148).
Data and Sample collection
Subjects during pregnancy were asked to complete a questionnaire to obtain information including age, gestational week, height and weight, etc. Maternal spot urine was obtained at enrollment from 8:00 to 11:00 AM after an overnight fast during an antenatal hospital visit. Dried blood spot samples were obtained from neonatal heels and infantile fingertips at birth and 18-24 months old, respectively. Infantile length and weight were measured at birth and 18-24 months old, and head and bust circumstance were measured only at 18-24 months old. Z-scores were calculated by (X-M)/S, M represents Reference standard median by WHO, S represents standard deviation, the physical growth of infants was categorized based on criteria of Z-scores recommended by WHO, <-2 is extremely delayed, <-1 is moderately delayed, <0 is slightly delayed, >0 is above the normal level, >1 is well developed, >2 is extremely developed. All samples were frozen at -80°C for assay within 2 weeks.
Analysis of urine samples & Categorization of UIC
UIC was analyzed by Inductively Coupled Plasma Mass Spectrometry (ICP-MS, Thermo Fisher Scientific iCAPTMQ, Germany) in the Tianjin Key Laboratory of Environmental Nutrition and Population Health. Based on the distribution of maternal UIC and WHO criteria, pregnant women were divided into 4 groups: <100μg/L (moderate deficiency), 100-149μg/L (mild deficiency), 150-249μg/L (sufficiency), >250μg/L (above requirements) in this study.
Determination of heel/fingertip TSH
Infants’ heel/fingertip blood spot samples were collected on filter papers by skilled nurses at 72 hours after birth. All the samples were sent to the Tianjin Maternal and Child Health Center for analysis. Dried blood spot TSH was measured using dissociation-enhanced fluor-immunoassay.
Statistical analysis
All statistical analyses were performed using SPSS25.0 (IBM, Inc., New York, NY, USA), and Microsoft Excel (Win10 2016). The
Kolmogorov-Smirnov method was used to test data distribution normality. Normally distributed data were presented as mean ± SD, and skewed data are presented as median (interquartile range). Difference in basic information between boys and girls was analyzed by t test or Kruskal-Wallis test. Difference in Z-scores, fingertip TSH and UIC of infants were analyzed via oneway ANOVA or nonparametric test. Significance was set at P<0.05 (two-tailed).
RESULTS
Demographic characteristic of infants at 18-24 months
Totally, 469 mother-infant pairs were enrolled in this study. The UIC of mother during pregnancy were 161 (111, 251) μg/L, indicating adequate iodine nutrition according to the WHOrecommended criteria of the UIC in pregnant women (data not shown). The basic information of their neonates was described in Table 1. Birth weight in female neonates was lower than that in male neonates (P=0.001), and the length-for-age (LFA) and weight-for-length (WFL) Z-scores were lower than those in male neonates (P<0.05). The median TSH levels of neonates were 3.1 (1.7, 4.5) mIU/L, no difference was found between boys and girls.
Pregnant women were recalled back 18-24 months after delivery, the average age of infants was 20.9 ± 3.0 months. Present weight, head circumference and bust in girls were lower than boys (P<0.001), no difference in gender of present length was found (P=0.231), and the corresponding WFL Z-scores in girls was also lower, while the LFA Z-scores in girls was higher than boys (P=0.007), differed from the trend of present length. The UIC in infants at 18-24 months was 217 (121, 361) μg/L, indicating adequate iodine nutrition. Both UIC and fingertip TSH in infants were not found difference between boys and girls (P>0.05).
UIC-specific difference of physical growth of infants
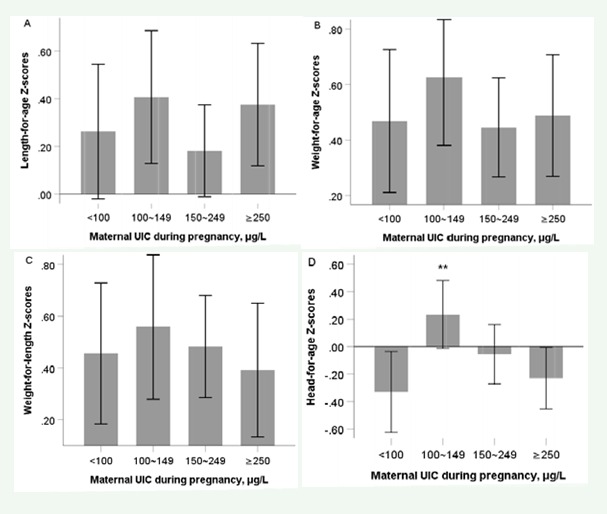
Based on the WHO-recommended criteria of the UIC during pregnancy, maternal UIC were divided into 4 groups (Table 2). No difference in birth length, weight, LFA Z-scores, weight-for-age (WFA) Z-scores and WFL Z-scores in neonates was found among different maternal UIC groups (all P>0.05). At 18-24 months after delivery, similar trends were found. Only head-for-age (HFA) Z-scores in UIC within 100-149μg/L during pregnancy was above 0, and it was highest (P=0.010), rather than 150-249μg/L, which
was recommended by WHO as the criteria of sufficient iodine nutrition during pregnancy (Figure 1).
Both heel-blood and fingertip TSH showed no differences among different UIC groups (P>0.05). Weak but no significant difference in UIC in infants at 18-24 months was found (P=0.661).
Difference of physical growth in infants with different neonatal TSH
No infants were diagnosed as congenital hypothyroidism. Take 4.0mIU/L as the cut-off point, neonatal TSH was divided into 2 groups (Table 3). Compared to neonates with TSH<4.0mIU/L, no difference of birth length, weight, LFA Z-scores, WFA Z-scores
and WFL Z-scores was found in neonates with TSH ≥4.0mIU/L (all P>0.05). At the age of 18-24 months, no significant changes also was found in length, weight, head circumference and bust in infants. LFA Z-scores in infants with neonatal TSH<4.0mIU/L was significantly higher than that in infants with neonatal TSH ≥4.0mIU/L (P=0.006), while no difference was found in WFA Z-scores, WFL Z-scores and HFA Z-scores between infants with neonatal TSH <4.0mIU/L and ≥4.0mIU/L (all P>0.05).
High neonatal TSH followed high fingertip TSH in infants at the age of 18-24 months (P<0.001), although fingertip TSH was within normal ranges. While, no impact was found in infants with neonatal TSH ≥4.0mIU/L on UIC in infants at 18-24 months was found (P=0.864).
DISCUSSION
Adequate iodine intake during pregnancy is vitally important to maintain the health of themselves and their offspring. A little much is too much, especially the iodine intake during pregnancy. Abnormal iodine nutrition during pregnancy has an adverse effect on the thyroid function of pregnant women and the brain development and physical growth of their infants [14,15]. UIC is most frequently used to evaluate the iodine nutrition of pregnant women and related criteria are defined by WHO/UNICEF/ ICCIDD. The UIC during pregnancy in the present study was 161 (111, 251) μg/L, suggesting sufficient iodine nutrition, which was consistent with previous study in Tianjin (16). While part of iodine-deficient pregnant women still exists. The effect of iodine deficiency during pregnancy on physical growth lacked concern evidence, a few studies reported negative effect [15,17], but no effects were also reported [18]. Our study aimed to evaluate the effect of iodine nutrition during pregnancy on physical growth of infants.
Boys’ physical and psychological development is earlier than girls’ at the same age [19-21] which was also shown in our study, especially in LFA Z-scores at 18-24 months, although no difference at birth. A Britain study showed no difference in infantile birth weight and length with various UIC levels during pregnancy [22], even in infants whose mothers were diagnosed with subclinical hypothyroidism or hypothyroidism [18,23]. Similar results were found in our study. While in other studies, for example, in Spain [24], a mildly iodine deficient country, compared with infants with maternal UIC <50μg/L, infantile weight was 189g heavier when their mothers’ UIC was within 100-150μg/L. Another study conducted in Wuhan, China, reported that when pregnant women had a UI/Creatinine higher than 500μg/g, their infantile weight birth weight was lighter than those infants with sufficient iodine nutrition (2833 vs 3007g) [25]. Serve iodine deficiency can result in cretinism, a series of irreversible and adverse outcomes of infantile development and growth. Restricted physical growth, as the most obvious symptom of cretinism, affects the entire life cycle of infants [26]. At 18-24 months after delivery, significance was only found in infantile HFA Z-scores among different maternal UIC levels during pregnancy. Only when the maternal UIC was within 100-149μg/L during pregnancy, the HFA Z-scores in infants was above zero, which may impact infantile intelligence, because the head circumference of infants was reported to be related to the distant brain development.
Congenital hypothyroidism can cause delayed psychological and physical development of infants, as well as children. No infants at birth were diagnosed as congenital hypothyroidism in our study due to the iodine nutrition in these areas being a mild-to-moderate deficiency, it’s not iodine deficient enough during pregnancy to lead to congenital hypothyroidism in their infants. LFA Z-scores in infants with heel-blood TSH <4.0mIU/L were higher than that with heel-blood TSH ≥4.0mIU/L. Neonatal TSH was reported to be positively related to maternal TSH during pregnancy [27], which correlated with their iodine nutrition. What’s more, higher neonatal TSH is followed with high TSH in children at 18-24 months, while this effects on their distant development is still uncertain. So more and deep studies are needed to confirm the influence of mild-to-moderate iodine deficiency during pregnancy on themselves and their infantile health.
In conclusion, even if the iodine nutrition during pregnancy was mildly deficient, it may affect their infantile physical development, especially the head circumference, which is related to brain development distantly. Further studies need to be conducted to clarify the effects of mild iodine deficiency during pregnancy on their infants. Surveillance should be continued on pregnant women all around the country.
There are limitations to our study. Firstly, our study only analyzed the effect of iodine-related factors during pregnancy, but other micronutrients were not included. Second, pregnant women and neonates recruited in this study were free of thyroid disease, which may lead to the neglect of some pregnant women who are susceptible to thyroid disease.
Table 1: describes the basic information of 469 newborns
|
|
Total (N=469) |
Boys (n=245) |
Girls (n=224) |
P |
|
Birth length, cm |
51.1 ± 1.8 |
51.2 ± 2.0 |
50.9 ± 1.7 |
0.079 |
|
Birth weight, kg |
3.4 ± 0.4 |
3.5 ± 4.4 |
3.3 ± 4.4 |
0.001 |
|
Birth LFA Z-scores |
0.81 ± 0.97 |
0.68 ±1.03 |
0.95 ± 0.88 |
0.003 |
|
Birth WFA Z-scores |
0.33 ± 0.99 |
0.33 ± 0.88 |
0.34 ± 1.09 |
0.968 |
|
Birth WFL Z-scores |
−0.58 ± 1.05 |
−0.39 ± 1.13 |
−0.80 ± 0.91 |
<0.001 |
|
Neonatal TSH, mIU/L |
3.1 (1.7, 4.5) |
3.1 (1.8, 4.4) |
3.0 (1.7, 4.5) |
0.788 |
|
Age, months |
20.9 ± 3.0 |
20.8 ± 2.9 |
21.0 ± 3.0 |
0.498 |
|
Present length, cm |
85.2 ± 4.1 |
85.4 ± 4.2 |
84.9 ± 4.1 |
0.231 |
|
Present weight, kg |
11.9 ± 1.5 |
12.2 ± 1.6 |
11.6 ±1.3 |
<0.001 |
|
Head, cm |
47.2 ± 1.6 |
47.6 ± 1.6 |
46.8 ± 1.5 |
<0.001 |
|
Bust, cm |
49.4 ± 2.8 |
49.9 ± 2.9 |
48.8 ±2.5 |
<0.001 |
|
LFA Z-scores |
0.34 ± 1.12 |
0.20 ± 1.23 |
0.48 ± 0.97 |
0.007 |
|
WFA Z-scores |
0.53 ± 1.03 |
0.53 ±1.15 |
0.53 ± 0.89 |
0.995 |
|
WFL Z-scores |
0.48 ± 1.14 |
0.60 ±1.25 |
0.35 ± 1.00 |
0.018 |
|
HFA Z-scores |
−0.17 ± 1.13 |
−0.26 ± 1.20 |
0.03 ± 1.04 |
0.064 |
|
Fingertip TSH, mIU/L |
1.4 (0.9, 1.9) |
1.4 (0.9, 2.0) |
1.4 (0.9, 1.9) |
0.778 |
|
UIC, μg/L |
217 (121, 361) |
241 (128, 374) |
191 (114, 325) |
0.121 |
|
Data were expressed as Mean ± SD or Median (P25, P75). TSH, thyroid stimulating hormone; UIC, urinary iodine concentration; LFA, length- for-age; WFA, weight-for-age; WFL, weight-for-length; HFA, head-for-age. |
||||
Table 2: describes the physical development of newborns with different urinary iodine levels.
|
|
UIC during pregnancy, μg/L |
P |
|
|||
|
<100 |
100-149 |
150-249 |
≥250 |
|||
|
Birth length, cm |
51.3 ± 1.7 |
51.0 ± 2.0 |
51.1 ± 2.0 |
50.7 ± 1.9 |
0.383 |
|
|
Birth weight, kg |
3.5 ± 0.4 |
3.4 ± 0.5 |
3.4 ± 0.5 |
3.3 ± .04 |
0.386 |
|
|
Birth LFA Z-scores |
0.89 ± 0.88 |
0.81 ± 1.08 |
0.82 ± 1.04 |
0.65 ± 1.02 |
0.514 |
|
|
Birth WFA Z-scores |
0.46 ± 0.88 |
0.35 ± 1.10 |
0.24 ± 1.10 |
0.23 ± 0.93 |
0.475 |
|
|
Birth WFL Z-scores |
−0.46 ± 0.98 |
−0.56 ± 1.13 |
−0.75 ± 1.10 |
−0.54 ± 1.07 |
0.355 |
|
|
Neonatal TSH, mIU/L |
3.7 (2.0, 4.9) |
2.6 (1.6, 4.0) |
3.1 (1.7, 4.7) |
3.5 (1.7, 4.5) |
0.195 |
|
|
Present length, cm |
85.8 ± 4.6 |
85.0 ± 4.3 |
84.5 ± 3.7 |
84.9 ± 3.9 |
0.291 |
|
|
Present weight, kg |
12.1 ± 1.6 |
11.9 ± 1.6 |
11.7 ± 1.3 |
11.7 ± 1.4 |
0.411 |
|
|
Head, cm |
47.0 ± 1.7 |
47.5 ± 1.7 |
47.2 ± 1.5 |
46.9 ± 1.4 |
0.098 |
|
|
Bust, cm |
49.3 ± 2.9 |
49.3 ± 2.5 |
49.3 ± 2.6 |
49.3 ± 2.9 |
0.999 |
|
|
LFA Z-scores |
0.26 ± 1.14 |
0.41 ± 1.29 |
0.18 ± 0.97 |
0.38 ± 1.19 |
0.526 |
|
|
WFA Z-scores |
0.47 ± 1.04 |
0.63 ± 1.13 |
0.45 ± 0.89 |
0.49 ± 1.02 |
0.652 |
|
|
WFL Z-scores |
0.46 ± 1.1 |
0.56 ± 1.29 |
0.48 ± 0.99 |
0.39 ± 1.2 |
0.818 |
|
|
HFA Z-scores |
−0.33 ± 1.19 |
0.23 ± 1.13 |
−0.06 ± 1.07 |
−0.23 ± 1.03 |
0.010 |
|
|
Fingertip TSH, mIU/L |
1.3 (0.8, 2.2) |
1.4 (0.9, 2.1) |
1.3 (0.8, 1.8) |
1.4 (1.0, 1.9) |
0.770 |
|
|
UIC, μg/L |
200 (115, 374) |
215 (99, 389) |
238 (140, 343) |
252 (159, 388) |
0.661 |
|
|
<100 |
14 (22.6%) |
18 (25.7%) |
16 (17.8%) |
11 (15.3%) |
0.398 |
|
|
Data were expressed as Mean ± SD or Median (P25, P75) or n (%). UIC, urinary iodine concentration; TSH, thyroid stimulating hormone; LFA, length-for-age; WFA, weight-for-age; WFL, weight-for-length; HFA, head-for-age. |
|
|||||
Table 3: describes the effects of different neonatal TSH levels on children's physical development.
|
|
Neonatal TSH, mIU/L |
P |
|
|
<4.0 |
≥4.0 |
||
|
Birth length, cm |
51.3 ± 1.9 |
51.3 ± 1.7 |
0.951 |
|
Birth weight, kg |
3.4 ± 0.5 |
3.4 ± 0.4 |
0.259 |
|
Birth LFA Z-scores |
0.95 ± 0.99 |
0.96 ± 0.91 |
0.934 |
|
Birth WFA Z-scores |
0.41 ± 1.01 |
0.29 ± 0.95 |
0.275 |
|
Birth WFL Z-scores |
−0.67 ± 0.90 |
−0.85 ± 0.86 |
0.082 |
|
Present length, cm |
85.5 ± 4.1 |
84.7 ± 4.2 |
0.094 |
|
Present weight, kg |
12.0 ± 1.6 |
11.8 ± 1.4 |
0.517 |
|
head (cm) |
47.4 ± 1.6 |
47.0 ± 1.6 |
0.086 |
|
Bust (cm) |
49.7 ± 2.9 |
49.6 ± 2.6 |
0.702 |
|
LFA Z-scores |
0.51 ± 1.1 |
0.17 ± 1.04 |
0.006 |
|
WFA Z-scores |
0.6 ± 1.12 |
0.48 ± 0.89 |
0.305 |
|
WFL Z-scores |
0.45 ± 1.24 |
0.52 ± 0.96 |
0.595 |
|
HFA Z-scores |
0.07 ± 1.1 |
−0.17 ± 1.19 |
0.066 |
|
Fingertip TSH, mIU/L |
1.2 (0.8, 1.7) |
1.8 (1.4, 2.3) |
<0.001 |
|
UIC, μg/L |
230 (130, 366) |
241 (127, 366) |
0.864 |
|
Data were expressed as Mean ± SD or Median (P25, P75). TSH, thyroid stimulating hormone; LFA, length-for-age; WFA, weight-for-age; WFL, weight-for-length; HFA, head-for-age; UIC, urinary iodine concentration. |
|||
FUNDING
This work was supported by ?the ?National Natural Science Foundation of China (No. 81920108031, ?82230113 and 81330064).
ACKNOWLEDGEMENTS
We gratefully acknowledge all the participants in this study, along with Tianjin Maternal and Child Health Hospital, for their assistance in thyroid function measurements.
AUTHOR CONTRIBUTIONS
Wanqi Zhang conceived the study. Wen Wu, Yanting Chen, Wenxing Guo, Qi Jin, Naifan Zhang, Kexin Zhang, Ying Yang, and Ziyun Pan participated in this study and collected data. Ziyun Pan was responsible for the analysis of urinary and blood samples and quality control. Wen Wu, Yanting Chen, Wenxing Guo, Qi Jin, Naifan Zhang, Kexin Zhang and Ying Yang analyzed the data, wrote and reviewed the paper. Wanqi Zhang drafted the paper, all authors contributed to and approved the final draft of the manuscript.
Informed Consent Statement: Informed consent was obtained from all subjects involved in the study.
REFERENCES
- Cao XY, Jiang XM, Dou ZH, Rakeman MA, Zhang ML, O’Donnell K, et al. Timing of vulnerability of the brain to iodine deficiency in endemic cretinism. N Engl J Med. 1994; 331: 1739-44.
- Zimmermann MB, Jooste PL, Pandav CS. Iodine-deficiency disorders. Lancet. 2008; 372: 1251-62.
- Stinca S, Andersson M, Herter-Aeberli I, Chabaa L, Cherkaoui M, El Ansari N, et al. Moderate-to-Severe Iodine Deficiency in the “First 1000 Days” Causes More Thyroid Hypofunction in Infants Than in Pregnant or Lactating Women. J Nutr. 2017; 147: 589-95.
- Glinoer D. The regulation of thyroid function during normal pregnancy: importance of the iodine nutrition status. Best Pract Res Clin Endocrinol Metab. 2004; 18: 133-52.
- Zimmermann MB. Methods to assess iron and iodine status. Br J Nutr. 2008 ; 99: S2-9.
- Henjum S, Kjellevold M, Ulak M, Chandyo RK, Shrestha PS, Froyland L, et al. Iodine Concentration in Breastmilk and Urine among Lactating Women of Bhaktapur, Nepal. Nutrients. 2016; 8: 255.
- Assessment of Iodine Deficiency Disorders and monitoring their elimination: A guide for programs managers. 3rd ed. Geneva, Switzerland: World Health Organization. 2007.
- Liu P, Su X, Shen H, Meng F, Fan L, Liu S, et al. National iodine deficiency disorders: An analysis of surveillance data in 2011. Chin J Endemiol. 2015; 34: 181-5.
- Yang J, Zhu L, Li X, Zheng H, Wang Z, Hao Z, et al. Maternal iodine status during lactation and infant weight and length in Henan Province, China. BMC Pregnancy Childbirth. 2017; 17: 383.
- Huynh D, Condo D, Gibson R, Muhlhausler B, Ryan P, Skeaff S, et al. Iodine status of postpartum women and their infants in Australia after the introduction of mandatory iodine fortification. Br J Nutr. 2017; 117: 1656-62.
- Bath SC, Steer CD, Golding J, Emmett P, Rayman MP. Effect of inadequate iodine status in UK pregnant women on cognitive outcomes in their children: results from the Avon Longitudinal Study of Parents and Children (ALSPAC). Lancet. 2013; 382: 331-7.
- Hynes KL, Otahal P, Hay I, Burgess JR. Mild iodine deficiency during pregnancy is associated with reduced educational outcomes in the offspring: 9-year follow-up of the gestational iodine cohort. J Clin Endocrinol Metab. 2013; 98: 1954-62.
- Rebagliato M, Murcia M, Alvarez-Pedrerol M, Espada M, FernandezSomoano A, Lertxundi N, et al. Iodine supplementation during pregnancy and infant neuropsychological development. INMA Mother and Child Cohort Study. Am J Epidemiol. 2013; 177: 944-53.
- Li Y, Shan Z, Teng W, Yu X, Li Y, Fan C, et al. Abnormalities of maternal thyroid function during pregnancy affect neuropsychological development of their children at 25-30 months. Clin Endocrinol (Oxf). 2010; 72: 825-9.
- Pop VJ, Brouwers EP, Vader HL, Vulsma T, van Baar AL, de Vijlder JJ. Maternal hypothyroxinaemia during early pregnancy and subsequent child development: a 3-year follow-up study. Clin Endocrinol (Oxf). 2003; 59: 282-8.
- Guo W, Wang W, Jin Y, Chen W, Chen L, Lin L, et al. Trimester-Specific Thyroid Function in Pregnant Women with Different Iodine Statuses. Ann Nutr Metab. 2020; 76: 165-74.
- Charoenratana C, Leelapat P, Traisrisilp K, Tongsong T. Maternal iodine insufficiency and adverse pregnancy outcomes. Matern Child Nutr. 2016; 12: 680-7.
- Casey BM, Dashe JS, Wells CE, McIntire DD, Byrd W, Leveno KJ, et al. Subclinical hypothyroidism and pregnancy outcomes. Obstet Gynecol. 2005; 105: 239-45.
- Guihard-Costa AM, Grange G, Larroche JC, Papiernik E. Sexual differences in anthropometric measurements in French newborns. Biol Neonate. 1997; 72: 156-64.
- Rodriguez G, Samper MP, Ventura P, Moreno LA, Olivares JL, PerezGonzalez JM. Gender differences in newborn subcutaneous fat distribution. Eur J Pediatr. 2004; 163: 457-61.
- Liu J, Lynn R. Chinese sex differences in intelligence: Some new evidence. Pers Individ Dif. 2015; 75: 90-3.
- Snart CJP, Keeble C, Taylor E, Cade JE, Stewart PM, Zimmermann M, et al. Maternal Iodine Status and Associations with Birth Outcomes in Three Major Cities in the United Kingdom. Nutrients. 2019; 11: 441.
- Kim HJ, Cho YY, Kim SW, Kim TH, Jang HW, Lee SY, et al. Reference intervals of thyroid hormones during pregnancy in Korea, an iodinereplete area. Korean J Intern Med. 2018; 33: 552-60.
- Alvarez-Pedrerol M, Guxens M, Mendez M, Canet Y, Martorell R, Espada M, et al. Iodine levels and thyroid hormones in healthy pregnant women and birth weight of their offspring. Eur J Endocrinol. 2009; 160: 423-9.
- Chen R, Li Q, Cui W, Wang X, Gao Q, Zhong C, et al. Maternal Iodine Insufficiency and Excess Are Associated with Adverse Effects on Fetal Growth: A Prospective Cohort Study in Wuhan, China. J Nutr. 2018; 148: 1814-20.
- Pearce EN, Lazarus JH, Moreno-Reyes R, Zimmermann MB. Consequences of iodine deficiency and excess in pregnant women: an overview of current known and unknowns. Am J Clin Nutr. 2016; 104: 918S-23S.
- Zhang Y, Du C, Wang W, Chen W, Shao P, Wang C, et al. Effect of maternal and neonatal factors on neonatal thyroid stimulating hormone: Results from a population-based prospective cohort study in China. J Trace Elem Med Biol. 2018; 49: 151-6.