Tizanidine Toxicity Requiring High Dose Naloxone Infusion: A Case Report and Review of the Current Literature
- 1. Northwell Department of Emergency Medicine, North Shore University Hospital, USA
- 2. Northwell Department of Medical Toxicology, North Shore University Hospital, USA
Abstract
Tizanidine is a centrally acting imidazoline derivative with a novel mechanism of action, closely resembling clonidine in its structure and pharmacological mechanism. Tizanidine has Federal Drug Agency (FDA) approval for the use of muscle spasticity and/or musculoskeletal pain, however, sometimes used off label in the treatment of trigeminal neuralgia and tension headaches. Although clonidine and associated structurally similar compounds (e.g., brimonidine, guanfacine, and tetrahydrozoline) exposures are more reported, unintentional and intentional exposures of tizanidine are sparsely found in the literature, and many of these case reports and treatment are an extrapolation of clonidine overdose given their structural and pharmacologic resemblance.
Keywords
Tizanidine, Clonidine, Muscle spasticity
Citation
Heuser W, Rizvi K, Nogar J (2023) Tizanidine Toxicity Requiring High Dose Naloxone Infusion: A Case Report and Review of the Current Literature. J Pharmacol Clin Toxicol 11(1):1173.
INTRODUCTION
Tizanidine is a centrally acting imidazoline derivative with a novel mechanism of action, closely resembling clonidine in its structure and pharmacological mechanism. Tizanidine has Federal Drug Agency (FDA) approval for the use of muscle spasticity and/or musculoskeletal pain, however, sometimes used off-label in the treatment of trigeminal neuralgia and tension headaches. Although clonidine and associated structurally similar compounds (e.g., brimonidine, guanfacine, and tetrahydrozoline) exposures are more reported, unintentional and intentional exposures of tizanidine are sparsely found in the literature, and many of these case reports and treatment are an extrapolation of clonidine overdose given their structural and pharmacologic resemblance. Presentation of previous case reports regarding alpha-2 and imidazoline receptor agonists were similar to ours with the most common effects reported to be hypotension, miosis, somnolence, lethargy, and bradycardia [1,2]. Furthermore, symptomatic imidazoline exposure are oftentimes managed medically with naloxone, however there is a paucity of literature on high dose or continuous naloxone infusion, specifically in the setting of tizanidine toxicity. We describe a case report of a large tizanidine successfully treated with the use of high dose naloxone followed by a naloxone continuous infusion.
CASE
A Thirty-year-old female with a past medical history of depression (not on any medications) was brought in by EMS after an intentional ingestion of approximately 12 tablets of 4mg tizanidine two hours prior to arrival. Patient was brought to the Emergency Department (ED), where patient was noted to be lethargic and unresponsive, with a Glasgow coma score (GCS) of 10 (E2, V4, M4). Patient was slurring her words on interview and continued to fall asleep and was unable to answer questions appropriately. Aside for mental status changes and miosis, the physical exam was unremarkable. Initial vital signs were: heart rate (HR) 93 bpm, blood pressure (BP) 136/112 mmHg, respiratory rate (RR) 16 breaths per minute, oxygen saturation of 98% on room air, and Temperature 97.2°F. ECG revealed sinus bradycardia with a rate of 46 bpm, QT 462 milliseconds, and QRS of 82 milliseconds. Initial laboratory values drawn on presentation were notable for: glucose 138, pH (venous) 7.36, pO2 43 mmHg, pCO2 43 mmHg, and bicarbonate (HCO3) 23 meQ/L, with a measured oxygen saturation (venous) of 74.6. A clean catch urine drug screen and serum drug screen was negative with undetectable levels of acetaminophen, ethanol, or salicylates.
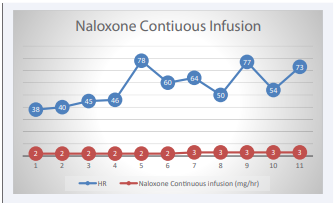
On reassessment (2 hours after presentation), the patient became bradycardic to 39 bpm and systolic BP (SBP) dropped to 90 mmHg with continued lethargy. Given altered mental status and lethargy, activated charcoal was held due to the risk of potential aspiration. Naloxone 4mg was administered intravenously, in which the patient immediately became more alert and no longer required physical stimulation for arousal. Hemodynamics remained bradycardic and hypotensive. Forty five minutes following naloxone (4mg) administration, the patient again became lethargic and an additional 4mg of intravenous naloxone was administered with appreciable response in mental status. Patient woke up to voice, however, intermittently became somnolent. Patient’s HR increased to 80 bpm, and SBPs to 100. Ninety minutes following naloxone IV patient became increasingly lethargic, bradycardic to 35 bpm, SBP to 105 mmHg, however, she was adequately maintaining her airway (oxygen saturation 98%, with equal chest rise). Given continued lethargy and altered hemodynamics, patient was admitted to the medical intensive care unit (MICU) and a naloxone continuous infusion was started at 2mg/hour for 6 hours and then increased to 3 mg/hour for an additional 5 hours (total of 11 hours) [Figure 1].
Figure 1: Naloxone continuous infusion with ass.
Patient remained in the MICU for hourly neurologic assessment, respiratory monitoring, and cardiac monitoring. The patient was discharged from the MICU following discontinuation of naloxone continuous infusion after patient regained baseline neurological mental status and HR changed from sinus bradycardia to normal sinus rhythm with a mean arterial pressure (MAP) >65.
DISCUSSION
As a central alpha-2 receptor and imidazoline receptor agonist, our patient presented in an expected fashion with altered mental status, bradycardia, and hypotension as a result of tizanidine toxicity. This toxidrome is consistent with its pharmacological properties as an alpha-2 receptor adrenergic receptor agonist which leads to inhibition of excitatory neurotransmitters (e.g., norepinephrine), via a negative feedback mechanism. The signs and symptoms of toxicity from alpha-2 agonist and imidazoline compounds are nearly identical to that of opiate intoxication with decreased mental status, hemodynamic compromise and miosis, prompting the use of naloxone as a reversal agent for this toxidrome. Previous case reports of tizanidine overdose treatments are an extrapolation of other imidazoline compounds (e.g., clonidine) that are well reported in the literature, and include the use of intravenous fluids, atropine, naloxone, and vasopressors [3,4]. Although these treatments are transiently effective, there is no one specific treatment that is highly effective. Naloxone is postulated to competitively inhibit the endogenous opioid receptor agonists (e.g., endorphins) that is released following imidazoline/alpha-2 agonist administration, thereby reversing the underlying central nervous system depression (CNS) and possibly improving hemodynamics through an increase in sympathetic tone [5-7]. Naloxone in high doses, however, has been well described, with no current reported cases utilizing a continuous infusion naloxone infusion for tizanidine which has been reported with structurally similar compounds like clonidine, with varying degrees of success. Although mechanistically novel, the use of naloxone to is not an antidote and high-quality supportive care should be performed when resuscitating these patients as there are notable case reports that describe naloxone’s inefficacy in reversing the myriad of symptoms associated with this toxidrome [8,9].The minimal and short-lived response to lower doses of naloxone (<10mg) utilized in our patient and subsequent improved response with higher or repeated dosages of naloxone is consistent with previous reports of naloxone non-responders in the setting of alpha-2/imidazoline overdose [10,11]. However, the response of imidazoline exposure to continuous naloxone infusion has been variable in previous literature, with even more limited evidence for tizanidine exposure describing only high dose naloxone with subsequent continuous infusion [Table 1].
Table 1: Case reports of Tizanidine overdose including intervention and subsequent response/disposition.
| Case Report | Intervention | Response/Disposition |
| 27-year-old male; 48mg ingestion of tizanidine13 | 10mg IV bolus of naloxone | Unremarkable ICU course followed by discharge at 38 hours post ingestion |
| 3-year-old male; unknown quantity of tizanidine ingestion14 | 0.05mg/kg of naloxone IV, followed by 0.1 mg/kg of naloxone within 40 minutes of initial dose | Monitored in the ICU and discharged home at 18 hours post ingestion |
Unlike previous case reports of tizanidine overdose, our patient had improvements in both neurological status as well as hemodynamics following naloxone administration. Improvements in level of consciousness, without significant improvements in hemodynamics are well reported in the literature for clonidine and other structurally similar imidazoline compounds. It is therefore reasonable, based on the success of our case as well previous case reports, that early high dose administration followed by continuous infusion should be considered in the setting of tizanidine overdose with neurological and hemodynamic alterations.
It should be noted that tizanidine, in contrast to clonidine, has a 20-fold greater affinity for the imidazoline receptor over the alpha-2 receptor [12].The implications of this greater affinity at the imidazoline receptor are not known but may cause a different, possibly more severe, clinical toxidrome than that of the alpha-2 agonist clonidine. The half-life elimination of tizanidine is short lived and reported to be around 2 hours, however, alterations in the pharmacokinetic/toxicokinetic profile were undeniably seen in our patient.
As noted in the aforementioned case description, our patient required multiple doses of naloxone in addition to a prolonged continuous infusion to maintain an appropriate mental status and perfusion status. At the time of our case report, there are no documented cases of tizanidine toxicity being successfully managed with naloxone infusion, despite the structural similarities to an imidazoline compound such as clonidine. Although this is one limited case report, utilization of naloxone infusion for tizanidine toxicity needs further investigation. This case report is an important example of the success of naloxone in the treatment of tizanidine poisoning and we feel that consideration for high dose of naloxone (e.g., 10mg) with a low threshold to start a continuous infusion, soon thereafter is an appropriate consideration in tizanidine toxicity. Although this case is the first reported successful case of the use of naloxone infusion to reverse the toxidrome of tizanidine, further studies are needed to confirm the role of naloxone continuous infusion in both the adult and pediatric population.