Hazard Analysis and Critical Control Point (HACCP) Plan for Carbonated Soft Drinks Plant
- 1. Department of Food Engineering, Faculty of Agricultural Engineering and Technology, University of Agriculture, Pakistan
- 2. Department of Food Science and Technology, Government College Women University, Pakistan
Abstract
Carbonated soft drinks are amongst the most consumable drinks around the world. The objective of this study was to design Hazard Analysis and Critical Control Point (HACCP) plan for soft drink production plant based on real time conditions in the beverage industry located in Faisalabad, Punjab, Pakistan. The model was developed to ensure the food safety of whole plant using the seven principles of HACCP and other generic models of HACCP using different approaches. Eight-member HACCP team was involved and HACCP chart, verification procedures and record-keeping were initiated. Only one CCP was identified during the production of carbonated soft drink. This research didn’t include the overview of production setup. Based on the results of this study, the authors recommend implementation of HACCP system in all food facilities.
Keywords
HACCP, CSD, Soft drinks plant, CCP, Food Safety
INTRODUCTION
HACCP is a short form for the Hazard Analysis Critical Control Point [1]. This system was developed to ensure pathogen-free foods. It provides accurate control measures & precautions to be followed for each step during whole process. The first portion of this system deals with the principles of the Hazard Analysis and Critical Control Point (HACCP) adopted by the Codex Alimentarius Commission. The guidance for application of the system are provided in second portion keeping in view that the details of application may vary depending on the circumstances of the food operation [2]. The researchers and specialists from The Pillsbury Company, The Natick Research Laboratories, and the National Aeronautics and Space Administration in USA were the pioneers to develop HACCP in late 1950s [3].
In modern era, food quality & food safety is compulsory for every industry and HACCP has made progress toward becoming synonymous with food safety [4]. HACCP is a tool that can be used for reducing the risks of food safety failure. All types of possible hazards are consider as a part of HACCP system, these include Biological, Physical and Chemical hazards [5]. The HACCP program ensures safety of products because potential hazards that may occur at any stage of processing are anticipated, evaluated, controlled and prevented. Processing plants are required to have a HACCP plan for each product [6]. HACCP framework requests a high initial investment just to get it implemented. The basis of this system requires additional resources for staff training, gear and additional provisions, as well as technical support [7].
Beverage industry is one of the renowned industry of modern era. Soft drinks are consumed a lot in every part of world. Due to high demand, the technology to produce soft drink also demanded a lot. Currently the several business groups are going to develop and invest in this field due to high demand.
Microbial hazards within soft drinks can be: (1) spoilage, done by general micro-organisms; (2) food poisoning done by pathogens [8]. The main ingredients and composition of soft drinks consists of water, fruit materials, sweeteners, flavorings, colorings, preservatives as well as other components [9]. Many micro-organisms are present in soft drinks due to contaminants, but few can survive within the acidic and low oxygen environment. Yeasts are the significant groups associated with spoilage of soft drink. Spoilage can occur as the metabolic by-products grow, for example, CO2 , acid, and tainting compounds [10]. The objective of this study was to develop a comprehensive, long term HACCP plan for beverages plant. So, by implementing the best plan in real time conditions at beverages plant can reduce the occurrence of hazards. This will identify health hazards and establish strategies to prevent, eliminate, or reduce the occurrence of physical, biological, or chemical hazards.
MATERIALS AND METHODS
This study was conducted at PepsiCo registered as name of Punjab Beverages Private Company Ltd. in Faisalabad, Punjab, Pakistan. This plant is categorized as large-scale plant for its production capacity. This plant has 700 workers, working in three shifts to produce average of 50,000 carbonated soft drink bottles per day. The objective of reforms was to expand company’s market. For that purpose, company planned effective quality system to ensure safe, healthy and best quality products.
The researchers spent four weeks in carbonated soft drink plant to observe and made hypothesis of final product. Employees and operators monitored the quality control in order to develop brief HACCP plan according to setup and processing in plant.
Collected Data of each step was analyzed during whole process which include raw material, packing until dispatch from plant, including all other procedures. Rest of the data was collected from tests, laboratory analysis records and from management.
Research Method
The research didn’t base on quantitative approach. The objective of this research was to develop HACCP plan for possible implementation in real time conditions. The study coordinated the qualitative approach that suggest inductive process directed by conceptual framework, driven more by itself hence there is no statistical data [11]. Subjective research was exploratory and open minded which was pertinent to this investigation [12].
Research Approach
The researchers developed a HACCP plan for carbonated soft drink based on the setup and processing in plant in order to enhance the quality of finished product. The HACCP plan was based on seven principles. These principles include risk analysis, CCP identification, setting critical limits, monitoring protocols, corrective actions, verification techniques, record-keeping, and documentation. In this study, models [13,14], guidelines [15,16], requirements [17] and record keeping related to HACCP was studied and designed.. This model was developed from NACMCF [18] that was adopted August 14, 1997 worldwide (FOODS).
The decision matrix tree is utilized to recognize Critical Control Point (CCP) for procedure [19].
RESULTS
This HACCP plan was designed on basics of seven principles of HACCP and by interpreting several models, to suit real time conditions of carbonated soft drink plant.
Prerequisite Program
The first step to design a HACCP plan was to bring all current essential prerequisite programs under the roof of HACCP and provide them a typical guidance about accomplishing zero deformities in finished product to guarantee that there was no health concern in the finished product. All prerequisite programs executed according to codex general principles of food hygiene and good manufacturing practices to bring hygienic food by establishing the essential condition during production.
Structure and Layout of Plant
The premises were designed in well manner structure that allow GMP, good food hygiene practices and prevent from accumulation of water. The design of building was in such a way that it mainly focused to prevent cross contamination. The base of building was sloped and drained. Although the corners and joints of the building were not well in form and it’s difficult to clean them. In some areas of the plant there were neither slope nor draining system, the water accumulated there. The walls of the plant were dust, Incest & rodent free. Although there were few cracks and gaps in some parts of wall that made it unsafe, it could be habitat for insects and rodents.
The glass covered doors and windows with aluminum finish are used throughout the plant. The air curtains were used specially on testing laboratories. The exhaust fans were used for proper air flow and prevent the building from heat accumulation and maintain relative humidity. The professional cleaners cleaned the plant on daily basis, the water accumulation and floor was cleaned after every shift.
Personal Hygiene
Personal hygiene has vital importance in every industry, it plays the role of back bone for production of finished safe food. The personal hygiene was followed properly by staff, but labor’s personal hygiene was improper. Although labor wore uniform but didn’t use net caps and gloves that might be source of contamination.
Equipment
The food grade material was used for equipment, such as stainless steel that could easily cleaned and maintainable. Each equipment checked after each shift to ensure proper working, smooth running, free of cracks and dents.
Pest Control
Pest control exercises were done through the professional teams available in state. In this modern era the pest control activities are carried out to prevent and control the pest, rather than to prevent pest as in traditional manners. This eliminated a huge risk of insects and rodent from spoiling the product.
Water supply
The best quality treated water was supplied for carbonated soft drink plant at desired temperature and parameters to ensure the good taste of drink.
Storage
The specific temperature was not required to store soft drinks. The storage was done at room temperature, but chilling before consumption was highly recommended.
Sanitation
The cleaning of area and drainage of water from warehouse were done on regular basis to ensure the safe and healthy finished product.
APPLICATION OF HACCP
Product Description
Product description defines the product completely with respect to applicable safety information such as: composition, intrinsic attributes, physical/chemical structure (including Aw, pH, etc.), shelf life, target market, and storage conditions and method of distribution [2] (Table 1).
|
Table 1: Carbonated soft drink product description. |
|
|
Product Description: |
Carbonated Soft Drink-Cold Processing |
|
Product Name(s) or SKU: |
Pepsi, 7up, Mirinda, Mirinda Green Cream Mountain Dew, Sting Berry Blast. Pepsi Diet, 7up Free, 7up Diet,7up mint RGB (Returnable Glass Bottle): 250 ml & 240 ml CAN: 250ml, 300ml PET:300ml, 345ml, 500ml, 1000ml, 1500ml, 1750ml, 2250ml. |
|
Intrinsic Product Attributes: (Food Safety Characteristics) - Including Ingredients |
Treated Water, CO2, Sugar-Sucrose Syrup, Sodium Benzoate, Citric Acid, Caffeine, PI flavors (Pepsi, 7up, Mirinda, Mountain Dew, Sting Berry Blast, Pepsi Diet, 7up Free, 7up Diet,7up Mint, Mirinda Green Cream) (Acidic) |
|
Customer/Consumer Use: |
Ready to use No cooking/processing required |
|
Target Market: |
Teenagers and Adults |
|
Vulnerable Consumer: |
Diabetic Patients (except 7up free, Pepsi Free and Diet Products like 7up Diet and Pepsi Diet), Carrying mothers (Sting, Dew, Pepsi Only) |
|
Special Distribution & Storage Control: |
Storage at Ambient temperature, under shed, protect from direct sunlight. |
|
Shelf Life: (If Applicable) |
PET = 6 months Sugar Free (7up & Pepsi) = 6 months Glass = 9 months Can = 6 months Can Diet = 6 months Can Sting BB = 1 year |
|
Label Instructions: |
Product Name, Brand Name, Name and Address of the Manufacturer, Net Content, Nutritional facts, Caloric Contents of Products, Ingredients, Serving instructions, Storage Instructions, Production Date and Expiry Date |
|
Method of Distribution: |
Delivery in Clean Covered vehicles having cover on it. |
Characteristics (Table 2)
|
Table 2: Characteristics relevant to food safety. |
|
|
Characteristics Relevant to Food Safety |
Carbonated Soft Drink |
|
Physio |
7up: As is brix:10.65± 0.2, CO2 PET:3.25-4.24, CO2 RGB:3.45-3.94, TA:22.36-24.70 Pepsi: As is brix:10.45± 0.2, CO2 PET:3.15-4.42, CO2 RGB;3.35-3.84, TA:10.90 Mountain Dew: As is brix:11.85± 0.2, CO2 PET:2.75-3.84, CO2 RGB;2.95-3.45, TA:18.91-21.02 Mirinda Orange: As is brix:12.95± 0.2, CO2 PET:1.35-2.44, CO2 RGB:1.5-2.05, TA:26.53-29.12 Mirinda Green Cream: As is brix:10.50± 0.2, CO2 PET:2.80-3.10, TA:6.94-7.36 7up Mint: As is brix:11.05± 0.2, CO2 PET:2.80-3.20, CO2 CAN:2.40-2.80 TA:38.18-40.54 Sting Berry Blast: As is brix:15.15± 0.2, CO2 PET:2.80-3.38, CO2 RGB:2.35-2.85, TA:50.35-53.04 7up Free :As is brix:0, CO2 PET:3.25-4.24, CO2 RGB;3.45-3.94, TA:25.50 Pepsi Diet :As is brix:0, CO2 PET:3.15-4.42, CO2 RGB;3.35-3.84, TA:13 |
|
Chemical |
N/A |
|
Microbiological |
Yeast ≤ 15/100mL, Mold ≤ 15/100mL, Aciduric Bacteria < 30/100mL TPC < 100 cfu/ml Coliform < 10 cfu/ml |
Intended Use (Table 3)
|
Table 3: Intended use of carbonated soft drinks. |
|
|
Queries related to product use |
Carbonated Soft Drink |
|
Will the product be cooked or heated by the consumer? |
No |
|
Will the product need specific storage until consumed? |
Yes, Stored at ambient temperature at covered places away from direct sunlight. |
|
Is the product likely to be mishandled by the consumer? |
Yes, Poor handling can result in cross contamination. |
|
Are there any vulnerable groups in the target market? |
Yes, sugar and caffeine based CSD, kids carrying mothers and diabetic patients |
|
Are there any specific allergens? |
No |
|
Are there any allergen claims made? |
No |
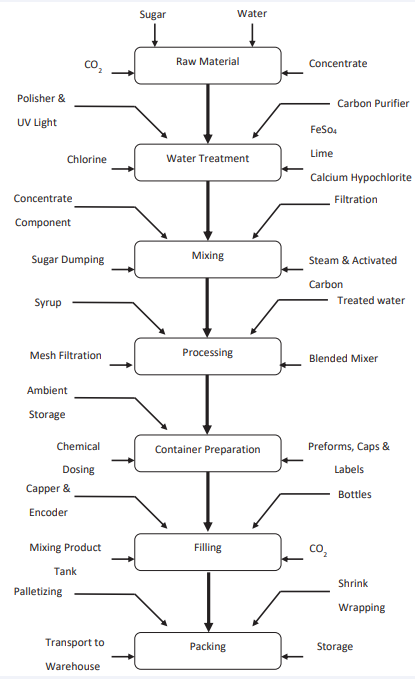
Flow Diagram
Figure 1 Flow Diagram.
Hazards Analysis and Critical Control Point (CCP) Determination (Tables 4-7)
|
Table 4: Severity determination level of hazards and effect of hazards. |
||
|
Severity Determination |
||
|
Severity If control fails: |
Effect of severity if hazard occurs |
Multiplier |
|
Little Damage |
Consumer disappointed. |
1 |
|
Damage |
Illness at home. Medical treatment not necessary. |
2 |
|
Serious Damage |
Minor medical treatment may be necessary (for example a broken tooth). |
3 |
|
Very Serious Damage |
Illness at home with medical treatment necessary. |
4 |
|
Disaster serious illnes |
Hospitilization Require |
5 |
|
Catastrophic |
Death |
6 |
|
Table 5: Probability of risk determination level of hazard. |
||
|
Risk/Likelihood Determination |
||
|
Probability of hazard occurring under current conditions |
Likelihood That Hazard Could Occur |
Multiplier |
|
Almost Impossible/Improbable |
No history of it occurring in facility or across the food and beverage industry |
1 |
|
Un-Likely/Remote |
Very occasional, has been known to occur in the facility or in a similar industry |
2 |
|
Small Risk/Possible |
Could be an isolated event that results after manual operations – would expect to happen occasionally in a year |
3 |
|
Likely/Probable |
Product or operational factors that can be expected to be present – could happen a number of times in the year |
4 |
|
Certain/Frequent |
Product or operational factors that are always present that the process is expected to control – occurs repeatedly |
5 |
|
Table 6: Severity determination of hazard. |
|
|
Risk/Severity determination |
|
|
Risk Score |
Risk Level Description |
|
1 – 5 |
Low risk - Probably controlled by a Prerequisite Program. Check nature of problem against existing pre-requisite programs. |
|
6 – 9 |
Some risk. Control by a Prerequisite Program. |
|
10 – 16 |
Elevated Risk. Use Codex decision tree for hazards. Definitely a Prerequisite Program, may be a CCP. |
|
16 + |
Significant risk! Likely to be a Critical Control Point (CCP). Use Codex decision tree for hazards. Initiate special HACCP review. |
|
Table 7: Hazard Identification and analysis for CSDs. |
|||||||||||||||||||
|
Operations |
Type of Hazard |
Cause of Haz- ard |
Significance of Hazard |
Justification for Decision |
Control Measure |
||||||||||||||
|
Sever- ity of Haz- ard |
Likeli- hood of Hazard Occur- ring |
Risk |
Signifi- cant Haz- ard (Yes or No) |
|
|
||||||||||||||
|
1.1 Source Water |
Physical: Sand, Mud etc. |
Occurrence in ground water |
2 |
3 |
6 |
No |
Water Treatment Process In Place |
Water Treatment System as Per PEPSICO guidelines and annual testing through external Lab |
|||||||||||
|
|
Chemical: Heavy Metals |
Carried with ground water |
2 |
2 |
4 |
No |
Water Treatment Process In Place |
Water Treatment System as Per PEPSICO guidelines and annual testing through external Lab |
|||||||||||
|
|
Biological: Presence of Total Coliform, E.coli |
Carried with ground water |
4 |
2 |
8 |
No |
Water Treatment Process In Place |
Water Treatment System as Per PEPSICO guidelines and annual testing through external Lab |
|||||||||||
|
1.2 Sugar |
Biological: Presence of TC, Mold, yeast |
From supplier process |
6 |
1 |
6 |
No |
Controlled by supplier. Certificate of analysis on delivery and RM tested. |
Supplier Quality Assurance Program. |
|||||||||||
|
Physical: Presence of FB e.g. metal filings. |
From Supplier drier process. |
3 |
1 |
3 |
No |
Controlled by supplier. CoA on delivery and RM tested. RM specifications in place. Filtration step later in process will address hazard. |
Supplier Quality Assurance Program, Filtration. |
||||||||||||
|
Chemical: Heavy Metals |
From supplier process |
3 |
1 |
3 |
No |
Controlled by supplier. Certificate of analysis on delivery. |
Supplier Quality Assurance Program. |
||||||||||||
|
1.3 Concentrate |
Biological: Presence of TC, Mold, yeast |
From supplier process. |
6 |
1 |
6 |
No |
Controlled by supplier (Pepsico). RM specifications in place. pH too low to sustain pathogen growth. |
Pepsico Quality Assurance Program. |
|||||||||||
|
Physical: Presence of FB e.g. metal filings. |
From supplier process. |
3 |
1 |
3 |
No |
Controlled by supplier (Pepsico). RM specifications in place. Filtration step later in process will address hazard. |
Pepsico Quality Assurance Program. |
||||||||||||
|
Chemical: Presence of industrial grade components e.g. citric acid. |
From supplier process. |
4 |
1 |
4 |
No |
Controlled by supplier (Pepsico). All components are food grade. RM specifications in place. |
Pepsico Quality Assurance Program. |
||||||||||||
|
1.4 Preforms |
Biological: Presence of mold, TC |
Unhygienic handling in supplier proc- ess. |
1 |
1 |
1 |
No |
Controlled by supplier. Fully automated system with hygiene standards in place. RM specifications in place. Approved supplier. |
Supplier Quality Assurance Program. |
|||||||||||
|
Physical: Presence of FB e.g. metal filings. |
From supplier process. |
1 |
1 |
1 |
No |
Controlled by supplier. RM specifications in place. Approved supplier. |
Supplier Quality Assurance Program. |
||||||||||||
|
Chemical: Presence of toxic chemicals. |
From supplier process. |
1 |
1 |
1 |
No |
Food grade certificate. RM specification in place. Approved supplier. |
Supplier Quality Assurance Program. |
||||||||||||
|
1.5 Caps |
Biological: Presence of mold, TC |
Unhygienic handling in supplier proc- ess. |
6 |
1 |
6 |
No |
Controlled by supplier. Fully automated system. RM specifications in place. |
Supplier Quality Assurance Program. |
|||||||||||
|
Physical: Presence of FB e.g. hair. |
From supplier process. |
1 |
1 |
1 |
No |
Controlled by supplier. Fully automated system with hygiene standards in place. RM specifications in place. |
Supplier Quality Assurance Program. |
||||||||||||
|
|
Chemical: Presence of toxic chemicals. |
From supplier process. |
1 |
1 |
1 |
No |
Food grade certificate. RM specification in place. |
Supplier Quality Assurance Program. |
|||||||||||
|
1.6 Labels |
Biological: NILL |
|
- |
- |
- |
No |
Secondary Packaging. No hazard Exist |
|
|||||||||||
|
Physical: NILL |
|
- |
- |
- |
No |
Secondary Packaging. No hazard Exist |
|
||||||||||||
|
Chemical: NILL |
|
- |
- |
- |
No |
Secondary Packaging. No hazard Exist |
|
||||||||||||
|
1.7 Empty Cans and Ends |
Biological: NILL |
|
- |
- |
- |
No |
No hazard Exist |
Supplier Quality Assurance Program |
|||||||||||
|
Physical: Dust |
Due To dam- age secondary packaging damage |
1 |
1 |
1 |
No |
Not likely to occur |
|
||||||||||||
|
Chemical: NILL |
|
- |
- |
- |
No |
No hazard Exist |
|
||||||||||||
|
1.8 Shrink Wrap Film |
Biological: NILL |
|
- |
- |
- |
No |
No hazard Exist |
|
|||||||||||
|
Physical: NILL |
|
- |
- |
- |
No |
No hazard Exist |
|
||||||||||||
|
Chemical: NILL |
|
- |
- |
- |
No |
No hazard Exist |
|
||||||||||||
|
1.9 CO2 |
Biological: NILL |
|
- |
- |
- |
No |
No hazard Exist |
|
|||||||||||
|
Physical: NILL |
|
- |
- |
- |
No |
No hazard Exist |
|
||||||||||||
|
Chemical: Presence of toxic impurities. |
From supplier process. |
3 |
1 |
3 |
No |
Approved supplier used, food grade CO2, purity tested on delivery, ingredient specification available, CoA on delivery. |
Supplier Quality Assurance Program. |
||||||||||||
|
2.1 CO2 purity |
Biological: NILL |
|
- |
- |
- |
No |
No hazard Exist |
|
|||||||||||
|
Physical: NILL |
|
- |
- |
- |
No |
No hazard Exist |
|
||||||||||||
|
Chemicals: Presence of adulterating chemicals. |
From supplier process. |
4 |
1 |
4 |
No |
Controlled by supplier. CoA on delivery and CO2 tested for purity. RM specifications in place. |
Supplier Quality Assurance Program. |
||||||||||||
|
2.2 CO2 unloading |
Biological: NILL |
|
- |
- |
- |
No |
No hazard during unloading |
|
|||||||||||
|
Physical: Foreign Matter |
During con- necting pipe- line with tank line |
1 |
1 |
1 |
No |
Not likely to be occurred |
CO2 Filtration |
||||||||||||
|
Chemical: NILL |
- |
- |
- |
No |
No |
|
|
||||||||||||
|
3.0 Storage |
Biological: Growth of vegetative pathogens. |
Cross contam- ination from damaged packaging. |
2 |
1 |
6 |
No |
Good warehouse practices in place. Staff well trained and GMP being followed. |
GMP, training. |
|||||||||||
|
Physical: introduction of FB. |
From en- vironment through dam- aged packag- ing. |
3 |
1 |
3 |
No |
Good warehouse practices in place. Staff well trained and GMP and cleaning schedule being followed. |
GMP, GHP, training. |
||||||||||||
|
Chemicals: Introduction of excrements from pests. |
Cross contam- ination from pests through damaged packaging. |
4 |
1 |
4 |
No |
Integrated pest management system being followed. Good warehouse practices in place. Staff well trained and GMP and cleaning schedule being followed. |
Pest control, GMP, GHP, training. |
||||||||||||
|
4. Cl2 Dosing |
Physical: Foreign Particle |
From Incom- ing Bag |
2 |
2 |
4 |
No |
Manage through PRP |
Incoming inspection program |
|||||||||||
|
Chemical: Cl2 |
Chemical Con- tamination due to over dosing of Cl2.Impurities in Cl2 |
3 |
2 |
6 |
No |
Manage through PRP |
Carbon Purifier |
||||||||||||
|
Biological: NILL |
|
- |
- |
- |
No |
No biological hazard exist |
|
||||||||||||
|
4.1 - Raw Water Reservoir |
Physical: NILL |
Present in po- table water |
1 |
1 |
1 |
No |
Not likely to occur |
Filtration through Sand and micron filtration |
|||||||||||
|
Chemical: Cl2 |
Overdosing of Cl2 |
3 |
2 |
6 |
No |
Manage through PRP |
Carbon Purifier in next program |
||||||||||||
|
Biological: E. coli Coliform |
Low dosage of Cl2 |
3 |
2 |
6 |
No |
Manage through PRP |
UV |
||||||||||||
|
4. 2 Brine Solution |
Physical: Foreign Matter |
Incoming material and handling |
1 |
2 |
2 |
No |
Not significant hazard |
Incoming material inspection |
|||||||||||
|
Chemical: |
|
- |
- |
- |
No |
No significant hazard occurred |
|
||||||||||||
|
Biological: Total Coliform |
Micro Con- tamination during annual maintenance from air, area & personal handling. |
2 |
2 |
4 |
No |
Manage through PRP |
Micro analysis of water |
||||||||||||
|
4.3 Softener |
Physical: Foreign Matter |
Probably present in treated water |
1 |
2 |
2 |
No |
No likelihoods |
Back washing of softener and changing at defined frequency |
|||||||||||
|
Chemical: NILL |
|
- |
- |
- |
No |
No biological hazard exists |
|
||||||||||||
|
Biological: Total Coliform |
Micro Con- tamination during annual maintenance from air, |
2 |
2 |
4 |
No |
Manage through PRP |
Micro analysis of water |
||||||||||||
|
4.4 Chemical Dosing |
Physical: Foreign Particles |
From Incom- ing Bag |
2 |
2 |
4 |
No |
Manage through PRP |
Testing at defined frequency |
|||||||||||
|
Chemical: Ferrous and Lime |
Contamina- tion due to over dosing of chemical |
2 |
2 |
4 |
No |
Manage through PRP |
Testing at defined frequency |
||||||||||||
|
Biological |
|
- |
- |
- |
No |
No biological hazard exists |
|
||||||||||||
|
4.5 Buffer Tank |
Physical: |
|
0 |
0 |
0 |
No |
No significant hazard occurred |
|
|||||||||||
|
Chemical: High Chlorine concentration |
Overdosing |
3 |
2 |
6 |
No |
Manage through PRP |
?Chlorine concentration is maintaining through water analysis as per defined frequency (>1 ppm) ?Removal of total chlorine through Carbon Filter |
||||||||||||
|
Biological: E.coli |
Contamina- tion improper dosing |
3 |
3 |
9 |
No |
Manage through PRP |
?Chlorination (6-8ppm) is maintained in buffer tank. ?Microbiological monitoring at plant's laboratory. ?Hygienic conditions of area and personnel are being followed. |
||||||||||||
|
4.6 Carbon Purifier |
Physical: Foreign Particles, carbon particles |
|
2 |
2 |
4 |
No |
Manage through PRP |
Follow Backwash frequency, Use Potable water |
|||||||||||
|
Chemical: Cl2 |
Improper Back washing |
4 |
3 |
12 |
|
As there is no other step for removal of chlorine and Likelihood is lower |
OPRP-1 |
||||||||||||
|
Biological: Total Coliform |
Micro Con- tamination during annual maintenance from air, area & personal handling. |
2 |
2 |
4 |
No |
Manage through PRP |
Follow Sanitation frequency, Use Potable Water |
||||||||||||
|
4.7 Polisher |
Physical: Foreign Matter |
Probably present in treated water or person |
4 |
3 |
12 |
|
There is no filter this is a last point but likelihood is lower |
OPRP-2 |
|||||||||||
|
Chemical: NILL Biological: Total Coliform |
Micro Con- tamination during annual maintenance from air, area & personal handling. |
- 2 |
- 2 |
- 4 |
No No |
No biological hazard exist Manage through PRP |
Hygienic conditions of area and personnel are being followed. Disinfection of filters before installation. |
||||||||||||
|
4.8 UV |
Physical: NILL |
|
- |
- |
- |
No |
No biological hazard exist |
|
|||||||||||
|
Chemical: NILL |
|
- |
- |
- |
No |
No biological hazard exist |
|
||||||||||||
|
Biological: Total bacteria, coliform |
Present in portable water |
4 |
3 |
12 |
|
High acidic product. Likelihood is lower |
Lamp replacement at defined frequency and micro testing at defined frequency (OPRP-3) |
||||||||||||
|
5.0 Sugar Dumping |
Physical: Foreign matter, threads, stones |
Sugar Bags |
2 |
2 |
4 |
No |
Manage through PRP |
Filtration of syrup in next steps |
|||||||||||
|
Biological: Contamination of Sugar from personals |
Unhygienic condition |
2 |
2 |
4 |
No |
Manage through PRP |
Area GMP and Personnel Hygiene |
||||||||||||
|
Chemical: NILL |
|
|
|
|
No |
Not likely to be occurred |
Chemical control program |
||||||||||||
|
5.1 Steam |
Physical: NILL |
|
- |
- |
- |
No |
Not likely to be occurred |
No direct contact |
|||||||||||
|
Chemical: Boiler chemical |
Non-Food grade chemical |
2 |
2 |
4 |
No |
Manage through PRP |
No Chemicals Used |
||||||||||||
|
Biological: Microorganism |
From water due to inappropriate Filter |
1 |
2 |
2 |
No |
Manage through PRP |
Micro analysis of soft water |
||||||||||||
|
5.2 Filtration |
Physical: Suspended particle |
Cross Contamination |
3 |
2 |
6 |
No |
Manage through PRP |
Visual inspection of simple syrup after each filtration. |
|||||||||||
|
Biological: yeast, mold total count |
Filter Chocking |
4 |
2 |
8 |
No |
Not likely to be occurred |
Cleaning and Sanitation program |
||||||||||||
|
Chemical: NILL |
|
- |
- |
- |
No |
Not likely to be occurred |
Close circuit, Cleaning and Sanitation program |
||||||||||||
|
5.3 Cooling through Heat Exchanger |
Physical: NILL |
|
- |
- |
- |
No |
Not likely to be occurred |
|
|||||||||||
|
Chemical: Coolant Contamination |
Leakage |
3 |
1 |
3 |
No |
No direct contact |
Simple syrup inspection, maintenance program |
||||||||||||
|
Biological: NILL |
|
- |
- |
- |
No |
Not likely to be occurred |
Cleaning and Sanitation program |
||||||||||||
|
5.4 Concentrate Components |
Physical: Foreign Particles, paper, tape and polythene pieces |
Present in Cartons |
2 |
2 |
4 |
No |
Manage through PRP |
Filtration of final syrup |
|
Biological: NILL |
|
- |
- |
- |
No |
Not likely to be occurred |
|
|
|
Chemical: NILL |
|
- |
- |
- |
No |
Not likely to be occurred |
|
|
|
5.5 Mixing Tank |
Physical: Foreign Particles, paper, tape and polythene pieces |
During Mixing of Ingredients |
2 |
2 |
4 |
No |
Manage through PRP |
Filtration of final syrup |
|
Biological: Yeast, Mold & Total Coliform |
Air borne micro contamination & non-appropriate cleaning and sanitation of mixing tank. |
2 |
2 |
4 |
No |
Manage through PRP |
*Cleaning and sanitation of mixing tank. (PRP) * Personal Hygiene and area GMP (PRP) |
|
|
Chemical: NILL |
|
- |
- |
- |
No |
Not likely to be occurred |
Cleaning and Sanitation program |
|
6.1 CO2 Filtration |
Physical: NILL |
|
- |
- |
- |
No |
Not likely to be occurred |
|
||||||
|
Chemical: Impurities |
From Supplier |
3 |
2 |
6 |
No |
CO2 From Approved Suppler |
Annual Lab Analysis From Approved Supplier TOA for each delivery |
|||||||
|
Biological: NILL |
|
- |
- |
- |
No |
Not likely to be occurred |
Close circuit |
|||||||
|
6.2 De-aeration |
Physical: NILL |
|
- |
- |
- |
No |
Not likely to be occurred |
Close circuit |
||||||
|
Chemical: NILL |
|
- |
- |
- |
No |
Not likely to be occurred |
Close circuit |
|||||||
|
Biological: NILL |
|
- |
- |
- |
No |
Not likely to be occurred |
Close circuit |
|||||||
|
6.3 Final Syrup Mesh Filtration |
Physical: Foreign Particles, paper, tape and polythene pieces, Pump Rubber |
Escape of item due to inappropriate filtration |
4 |
4 |
16 |
|
This is last step for filtration |
Daily 100 mesh strainer inspection |
||||||
|
Chemical: NILL |
|
- |
- |
- |
No |
Not likely to be occurred |
|
|||||||
|
Biological: Micro organism Yeast & Mold |
Micro contamination during maintenance of pump from personal contact and from area. |
2 |
2 |
4 |
No |
Manage Through PRP |
Area and Personnel Hygiene |
|||||||
|
6.4 Syrup receiving & Proportioning |
Physical: Foreign Particles, paper, tape and polythene pieces, Pump Rubber Seals |
From Syrup and water |
2 |
2 |
4 |
No |
Filtration |
100 mesh strainer at syrup lines and 5 micron filter on air |
||||||
|
|
Chemical: Residue |
Contamination from residue from Changeover or sanitation |
2 |
2 |
4 |
No |
Verification after each CIP |
CIP SOP and Changeover Matrix |
||||||
|
|
Biological: NILL |
Yeast, Mold |
2 |
2 |
4 |
No |
Not likely to occur |
CIP SOP and Changeover Matrix |
||||||
|
6.5 Carbonation |
Physical: Foreign matter |
From supply lines and tanks |
2 |
2 |
4 |
No |
Filter are in place |
Purging of line, Filter maintenance |
||||||
|
Chemical: Impurities |
From Supplier |
2 |
2 |
4 |
No |
Incoming COA/COC TOA on each delivery |
Annual lab Analysis of CO2 |
|||||||
|
Biological: NILL |
|
- |
- |
- |
No |
Not likely to be occurred |
|
|||||||
|
6.6 Mixer Product Tank |
Physical: NILL |
|
- |
- |
- |
No |
Not likely to be occurred |
|
|
|
Chemical: NILL |
|
- |
- |
- |
No |
Not likely to be occurred |
|
||
|
Biological: Yeast, Mold |
Ineffective sanitation |
2 |
1 |
2 |
No |
Not likely to be occurred |
Sanitation and Micro Sampling |
||
|
7.1 Washer |
Physical: Cemented Bottles, Chipped bottles, Cracked Bottles & Bottles containing foreign matters (with sharp edges, stones, glass pieces), Other brand bottles |
Contaminated Container and improper pre wash inspection |
2 |
2 |
4 |
No |
Control Measures in place and effective |
Sorting of all such bottles by trained visual inspectors at first light & final light. Washing through washer |
|
|
Chemical: chemical residue |
Carryover along contamination |
2 |
2 |
4 |
No |
Control Measures in place and effective |
?Visual inspection of jets for alignment. ?Maintain required rinsing conditions including: •Pressure of jets >1.5 bar •Accurate alignment of jets. •Maintained pH 7-8 •Concentration of caustic >2.0 % •Contact Time: 7 minutes |
||
|
|
Biological: Survival of E. coli, Salmonella & Salmonella & another mold. |
From storage and handling |
3 |
3 |
9 |
No |
Control Measures in place and effective |
?Bottles are thoroughly washed & sanitized to ensure elimination of pathogens. Following conditions are maintained for cleaning sanitation of containers ?Visual inspection of jets for alignment •Temperature of water: 60-70 ?C •Concentration of caustic solution >2.0 % •Contact Time of caustic with container : 7 minutes •Additives •Microbiological analysis as per defined frequency •Mold test of washed container after washing |
|
|
7.2 Hopper Filling |
Chemical: NILL |
No food safety hazards were identified. |
- |
- |
- |
No |
No Significant Hazard Occur |
|
|
|
Biological: NILL |
Pest activity while feeding the hopper cover is open |
3 |
1 |
3 |
No |
GMP |
Pest control program implementation |
||
|
Physical: NILL |
Dust (uncleaned surface) + Packing Remains |
1 |
1 |
1 |
No |
GMP |
GMP and regular inspection |
||
|
7.3 Preform Lift conveyor |
Chemical: NILL |
|
- |
- |
- |
No |
No Significant Hazard Occur |
|
|
|
Biological: Mold, Yeast |
Pest activity while the cover is open |
3 |
1 |
3 |
No |
GMP |
Pest control program implementation |
||
|
Physical: Foreign Matters |
Dust (uncleaned surface) |
1 |
1 |
1 |
No |
Control Measures in place and effective |
GMP and regular inspection |
||
|
7.4 Preform Neck Camera Inspection |
Chemical NILL |
|
- |
- |
- |
No |
No Significant Hazard Occur |
|
|
|
Biological: Yeast, Mold |
Pest activity while the cover is open |
2 |
1 |
2 |
No |
GMP |
Pest control program implementation |
||
|
|
Physical: Foreign Matter |
Dust (uncleaned surface) |
1 |
1 |
1 |
No |
GMP |
GMP and cleaning by ionized air |
|
|
7.5 Compressed Air |
Physical: NILL |
|
2 |
0 |
0 |
No |
Control Measures in place and effective |
Filters in Place |
|
|
Chemical: NILL |
Oil Used for Lubrication |
2 |
0 |
0 |
No |
Control Measures in place and effective |
Oil free compressors are in use |
||
|
Microbiological: Yeast and Mold |
Environment |
2 |
1 |
2 |
No |
Control Measures in place and effective |
Environment and Empty PET Microbiology Testing Done |
||
|
7.6 Blowing Molds |
Chemical: Lubricants |
Lubrication |
1 |
1 |
1 |
No |
GMP and regular cleaning |
Food Grade greases are being used |
|
|
Biological: Mold, Yeast |
Pest activity while the cover is open |
1 |
1 |
1 |
No |
Control Measures in place and effective |
Pest control program implementation |
||
|
Physical: Foreign Matter |
Dust (uncleaned surface) |
1 |
1 |
1 |
No |
Control Measures in place and effective |
GMP and cleaning |
||
|
7.7 Bottle Guiding Star |
Chemical: Lubricants |
Lubrication |
1 |
1 |
1 |
No |
Control Measures in place and effective |
Food Grade greases are being used and below bottle neck |
|
|
Biological: Mold, Yeast |
Pest activity while the cover is open |
1 |
1 |
1 |
No |
Control Measures in place and effective |
Pest control program implementation |
||
|
Physical: Foreign Matter |
Dust (uncleaned surface)/ Rust on the grippers |
1 |
1 |
1 |
No |
Control Measures in place and effective |
GMP and cleaning |
||
|
8.1 Air Conveyors |
Chemical NILL |
|
- |
- |
- |
No |
Not likely to be occurred |
|
|
|
Biological: Yeast, Mold |
Pest activity while the cover is open |
1 |
1 |
1 |
No |
GMP |
Pest control program implementation |
||
|
Physical: Foreign Matter |
Dust (uncleaned surface)/ Rust on the guiding strip |
2 |
1 |
2 |
No |
GMP and design |
GMP and cleaning |
||
|
8.2 Base Cooling |
Physical: NILL |
|
|
|
|
No |
Not likely to be occurred |
||
|
Chemical: NILL |
|
- |
- |
- |
No |
Not likely to be occurred |
|||
|
Biological: NILL |
|
- |
- |
- |
No |
Not likely to be occurred |
|||
|
8.3 Labeling |
Physical: NILL |
|
- |
- |
- |
No |
Not likely to be occurred |
||
|
Chemical: NILL |
|
- |
- |
- |
No |
Not likely to be occurred |
|||
|
Biological: NILL |
|
|
|
|
No |
Not likely to be occurred |
|
||
|
8.4 Rinse |
Physical: Dust particles |
Poor rinsing |
1 |
2 |
2 |
No |
Control Measures are effective |
Rinse Pressure monitoring Rinse alignment monitoring |
|
|
Chemical: Residual Water |
|
1 |
2 |
2 |
No |
Control Measures are effective |
Residual Volume monitoring |
||
|
Biological NILL |
|
- |
- |
- |
No |
Not likely to be occurred |
|
||
|
8.5 Crown/closure Debagging and transfer to hopper |
Physical: NILL |
|
- |
- |
- |
No |
Not likely to be occurred |
|
|
|
Chemical: NILL |
|
- |
- |
- |
No |
Not likely to be occurred |
|||
|
Biological: NILL |
|
- |
- |
- |
No |
Not likely to be occurred |
|||
|
8.6 Filling |
Physical: Presence of sharp-edged glass fragments, Carrying of foreign particles from empty bottle inspection |
Its due to bottles bursting, Second occurs due to untrained bottle inspectors. |
3 |
3 |
9 |
No |
Control Measures are effective |
*Bottle failure Procedure *Training of bottle inspectors. |
|
|
|
Chemical: NILL |
No significant hazard occurs |
- |
- |
- |
No |
Not likely to be occurred |
|
|
|
|
Microbiological: Microbiological contamination (mold, yeast) |
Its due to Non- appropriate cleaning of containers Dirty filling equipment From air-borne micro-organisms. Personal contacts Dirty bottles due to poor inspection of bottles by untrained bottle inspectors. |
3 |
2 |
6 |
No |
Control Measures are effective |
Monitoring Washer parameters. Cleaning & Sanitation of process equipment’s (CIP). Area GMP and Personal Hygiene* Training of bottle inspectors. |
|
|
8.7 Capping |
Physical: Cap shoot particles & tap and paper pieces |
Its comes from cap cartons, improper cleaning |
3 |
2 |
6 |
No |
Control Measures are effective |
Cleaning & Maintenance of equipment as per schedule. Manual Cleaning of cartons before opening. *Training of filler operators. |
|
|
Chemical |
|
- |
- |
- |
No |
Not likely to be occurred |
|
||
|
Microbiological: Air borne microorganisms & Mold |
Its comes from personal and second due to improper cleaning |
2 |
2 |
4 |
No |
Control Measures are effective |
Area and machine GMP and personal Hygiene |
||
|
8.8 Date Coding |
Physical: NILL |
|
- |
- |
- |
No |
Not likely to be occurred |
|
|
|
Chemical: NILL |
|
- |
- |
- |
No |
Not likely to be occurred |
|
||
|
Biological: NILL |
|
- |
- |
- |
No |
Not likely to be occurred |
|
||
|
8.9 Bottle Inspection |
Physical: cracked and foreign matter |
bottle and machine contact |
2 |
2 |
4 |
No |
Control Measures are effective |
Bottles Inspectors training |
|
|
Chemical NILL |
|
- |
- |
- |
No |
Not likely to be occurred |
|
||
|
Biological: NILL |
|
- |
- |
- |
No |
Not likely to be occurred |
|
||
|
8.10 Warmer |
Physical: Bottles crack |
High temperature of water |
2 |
2 |
4 |
No |
Control Measures are effective |
Monitoring of Temperature |
|
|
Chemical: NILL |
|
- |
- |
- |
No |
Not likely to be occurred |
|
||
|
Microbiological: Mold, E.Coli |
|
- |
- |
- |
No |
Not likely to be occurred |
treated water used and micro sampling |
||
|
8.11 Washed Containers Visual Inspections |
Physical: Non- removed foreign matters, Glass particles from breakage of bottles due to thermal shock, damaged pockets, other brand bottles |
Improper cleaning and not removal by EBI and Prewash inspectors |
2 |
2 |
4 |
No |
Control Measures are effective |
Inspection of washed containers at light by trained & permanent visual inspectors. |
|
|
Chemical: oil, greasy, paint |
From washer |
2 |
2 |
4 |
No |
Control Measures are effective |
Inspection by trained visual inspectors |
||
|
Biological: air bone contamination |
From Handling and improper washing |
2 |
2 |
4 |
No |
Control Measures are effective |
Good cleaning and hygienic practices. Monitoring of air quality at defined frequency |
||
|
9.1 Case Packing |
Physical: NILL |
|
- |
- |
- |
No |
Not likely to be occur |
|
|
|
Chemical: Residual oil |
From Air |
- |
- |
- |
No |
Not likely to be occur |
|
||
|
|
Biological: Mold |
From sugar due to leakage of any previous product |
1 |
1 |
1 |
No |
Control Measures in place |
Leak can sort out |
|
|
9.2 Shrink Wrap |
Physical NILL |
|
- |
- |
- |
No |
Not likely to be occur |
|
|
|
Chemical NILL |
|
- |
- |
- |
No |
Not likely to be occur |
|
||
|
Biological NILL |
|
- |
- |
- |
No |
Not likely to be occur |
|
||
|
9.3 Palatizing |
Physical NILL |
|
- |
- |
- |
No |
Not likely to be occur |
|
|
|
Chemical NILL |
|
- |
- |
- |
No |
Not likely to be occur |
|
||
|
Biological NILL |
|
- |
- |
- |
No |
Not likely to be occur |
|
||
|
9.6 Storage |
Biological: Micro Growth TC, Yeast, Mold |
Cross contamination from damaged packaging. |
6 |
1 |
6 |
No |
Effective Control Measures in place |
Good warehouse practices in place. Staff well trained and GMP being followed MP, training. |
|
|
Physical: Introduction of FB. |
From environment through damaged packaging. |
3 |
1 |
3 |
No |
Effective Control Measures in place |
Good warehouse practices in place. Staff well trained and GMP and cleaning schedule being followed MP, GHP, training. |
||
|
Chemicals: Introduction of excrements from pests. |
Cross contamination from pests through damaged packaging. |
4 |
1 |
4 |
No |
Effective Control Measures in place |
Integrated pest management system being followed. Good warehouse practices in place. Staff well trained and GMP and cleaning schedule being followed MP, GHP, training. |
||
|
8.12 Seamer |
Physical: Dust can enter |
|
- |
- |
- |
No |
Not likely to be occurred |
|
|
|
Chemical NILL |
|
- |
- |
- |
No |
Not likely to be occurred |
|
||
|
Biological: Air bone contamination |
Due to improper sealing |
2 |
2 |
4 |
No |
Control Measures are effective |
Seamer Inspection |
||
Operations Pre-Requisite Program for Carbonated Soft Drinks (Table 8)
|
Table 8: OPRP plan for CSDs. |
|||||||||||
|
OPRP Plan for CSDs Lines |
|||||||||||
|
OPRP # & De- scrip- tion |
Food Safety Hazard to be control- led |
Control Measure |
Critical Limit |
Monitoring Procedures |
Correction |
Correc- tive Ac- tions |
Re- sponsi- bility & Author- ity (Who?) |
Records Of Moni- toring and Loca- tion |
|||
|
What |
How |
Fre- quency |
Who |
||||||||
|
Activity |
Method to be used |
How of- ten? |
Responsi- ble for moni- toring? |
Activity (What?) |
Activity (What) |
||||||
|
Acti- vated Car- bon Tank |
Chemi- cal: High Residual Chlorine |
Chlorine NILL after activated Carbon Tank |
NILL |
Chlo- rine moni- toring after A.C |
Colorim- eter |
At star- tup and every four hours |
Water Treatment operator |
Hold the pro- duction from last good check to current check random sam- ples and decide accordingly |
Carry out root cause analysis and take corrective actions |
Process Engi- neer |
Water treatment quality report |
|
5 Mi- cron Filter |
Physical: Sand/ Acti- vated Carbon particles |
Physical: Sand & Silt Parti- cles |
Pressure Drop < 5 PSI |
Pres- sure Drop Calcula- tion |
Visual monitor- ing |
Daily |
Water Treatment operator |
Hold the pro- duction from last good check to current check random sam- ples and decide accordingly |
Carry out root cause analysis and take corrective actions |
Process Engi- neer |
Filter change record Available in Water Treatment |
|
UV |
Biologi- cal: Colif- orm |
UV moni- toring and micro analysis |
Intensity >70 % Operating Hours < 8000 hours Total bacteria 50/100mL, col- iform bacteria (cfu) 0/100mL |
Micro- bial Count of treated water Mainte- nance Log of UV |
Visual monitor- ing/ Micro results |
UV pa- rameters monitor- ing once in 8hour shift and weekly micro analysis |
Microbiol- ogist Water Treatment Operator |
Hold the pro- duction from last good check to current check random sam- ples and decide accordingly |
Carry out root cause analysis and take corrective actions |
Process Engi- neer |
Micro- biology reports, 5 Step CIP re- ports, UV mainte- nance Log Available in Water Treatment |
CCP DETERMINATION FOR CARBONATED SOFT DRINK (TABLE 9)
Instructions [20]
Q-1 :Do Preventive approach Exist For BCP If Yes (Y), proceed
for Q2, if No (N),Is control compulsory? If No, not a CCP.
Q2: Does This Step Eliminate/Reduce The Likely Occurrence Of BCP Hazard To An Acceptable Lev1el? If No, Proceed for Q3, if Yes, that is CCP.
Q3: Could Unacceptable BCP Contamination Occur? If Yes, proceed for Q4, process or product, if No, not a CCP.
Q4: Will Subsequent Step Eliminate BCP Hazard? If No, CCP,
if Yes, not a CCP.
|
Table 9: CCP determination for CSDs. |
|||||||||
|
CCP Determination for Carbonated Soft Drink |
|||||||||
|
Process Step |
Hazard Type |
Control Measure |
Is the haz- ard com- pletely controlled by a pre- requisite program? |
Decision Tree Answers |
|
Justification of Decision |
|||
|
Q-1 Do pre- ventable control measures exist? |
Q-2 Is the step specifically designed to eliminate or reduce the likely occurrence of hazard to an ac- ceptable level? |
Q-3 Could con- tamination with identi- fied hazard oc- cur in excess of accept- able level or could these increase to unacceptable levels? |
Q-4 Will a subse- quent step eliminate identified hazard or reduce likely occurrence to accept- able level? |
CCP (Yes/No) , CCP# |
|||||
|
Final Syrup Strainer |
Physical: Foreign Object s |
Yes Filtration with the filter of mesh size 100 |
NO |
Yes |
Yes |
Yes |
No |
YES CCP1 |
The inline 100 mesh filter positioned as the last filtration step at the closest point prior to filling specifically designed to control the identified foreign object hazards. As Syrup Room is the last manual Handling Operation |
CCP Control Chart (Table 10)
|
Table 10: CCP control chart for CSDs. |
||||||||||||
|
Proc- ess Step / CCP# |
CCP # |
Type of Hazard |
Control Meas- ure |
Critical Limits |
Monitoring Procedures |
Correc- tion |
Correc- tive Ac- tions |
Responsibil- ity & Au- thority (Who?) |
Records Of Monitor- ing and Location |
|||
|
What |
How |
Frequen- cy |
Who |
Activity (What?) |
Activity (What?) |
|
|
|||||
|
Activity |
Method to be used |
How of- ten? |
Respon- sible for monitor- ing? |
|||||||||
|
Final Syrup Strain- er |
CCP # 1 |
Physi- cal: Pres- ence of foreign matter |
Flltra- tion Through inline 100 mesh filter |
Absent, Filter intact and free of foreign matter |
Mesh filter (located prior to filling). |
Visual in- spection of the filter/ screen for integrity, assembled correctly and of the correct size for the prod- uct |
Daily |
Syrup Room Op- erator |
1.If filter/ screen is not present, intact or the cor- rect size during verifica- tion, check on hold, stop the proc- ess, place the ma- chine "out of order". 2.Notify designated quality employee. 3.Conduct risk as- sessment to evaluate the likeli- hood of the hazard. 4.Replace damaged filter with a function- al filter. |
Carry out root cause analysis as correc- tive and preven- tive action program |
Process Engineer/ Syrup Room Operator/ FSTL |
Strainer Inspection Record Available in Syrup Room |
CONCLUSION
The safe and healthy food along with wholsomeness is highly demanded in this modern era of development and improvement in food industry. For the purpose of production and distribution of safe food,the industries are implementing safety mangements systems. This HACCP model was developed to improve the safety and quality of the carbonated soft drink plant. Based on seven principles of HACCP system, this model was developed step by step. The item depiction was utilized to warn the consumer about the potential perils in the finished items.Then, during process the prevention measures elaborated together with the potential control points of the hazards. The critical control points were determined by use of decision trees. Finally, by taking in view the seven principles of HACCP the control chart, critical limits, monitoring and corrective action was developed. One CCP was found in the processing of Carbonated soft drink. That was final syrup stainer.
REFERENCES
- Bardic AJDF. HACCP ready. 2001; 184: 6
- Food, C. A. C. J. and R. Agriculture Organization. Hazard analysis and critical control point (HACCP) system and guidelines for its application. 1997; Annex to CAC/RCP 1-1969, Rev. 3.
- Surak, J. G. J. F. Q. M., February/March. The Evolution of HACCP–A perspective on today’s most effective food safety system. 2009.
- 97/13A, C. A. C. J. A. Principles for the establishment and application of microbiological criteria for foods. 1996.
- Mortimore, S. Carol A. Wallace. HACCP: A practical approach, Springer Science & Business Media. 2013.
- Julie K Northcutt, Scott M. Russell. General guidelines for implementation of HACCP in a poultry processing plant. 2010.
- Yasmine Motarjemi, Fritz Käferstein. Food safety, Hazard Analysis and Critical Control Point and the increase in foodborne diseases: a paradox?. 1999; 10: 325-333.
- Wareing P, Davenport RR. Microbiology of soft drinks and fruit juices. 2005; 279-299.
- Philip R. Ashurst, Robert Hargitt, Fiona Palmer. Soft drink and fruit juice problems solved, Woodhead Publishing. 2017.
- Hocking A. Soft drinks, cordials, juices, bottled water and related products. 2001; 93-94.
- Mihas P. Qualitative data analysis. Oxford Research Encyclopedia of Education. 2019.
- Michael Quinn Patton. How to use qualitative methods in evaluation, Sage. 1987.
- Burson D. Hazard Analysis Critical Control Point (HACCP) Model for Frankfurters. 2015; NE 68583-0908.
- Quaye S. Development of a HACCP Plan for a Model Cold Pressed Virgin Coconut Oil Industry in Ghana. 2018.
- Pineiro M. Manual on the Application of the HACCP System in Mycotoxin Prevention and Control. Food & Agriculture Org, 2001.
- FAO, WHO Guidance to Governments on the Application of HACCP in Small and/or Less Developed Businesses, FAO Food Nutr Pap. 2006; 86: 1-74.
- Rahul Soman, Meera Raman. HACCP system–hazard analysis and assessment, based on ISO 22000: 2005 methodology. 2016; 69: 191-195.
- Robert L Buchanan. NATIONAL ADVISORY COMMITTEE ON MICROBIOLOGICAL CRITERIA FOR FOODS. J Food Prot. 1997; 60: 1417-1419.
- Alimentarius, C.J. R. I. C. o. P. G. P. o. F. H.CAC/RCP 1-1969, Rev. 4,2003; 3.
- Roepstorff A. Food and Agriculture Organization of the United Nations. 1998.