Occurrence and Health Risk Assessment of Tropane Alkaloids (Atropine and Scopolamine) in Cereal-Based Foods Consumed by Children and Adults in N’djamena, Chad
- 1. Laboratory of Pharmacology and Toxicology, Department of Biochemistry, University of Yaounde 1, Cameroon
- 2. Agri-Food Safety and One Health Agency (AFS1HA), Cameroon
Abstract
Tropane alkaloids (TAs), mainly atropine and scopolamine, are toxic, plant-derived substances produced as secondary metabolites and which are primarily found in Datura Stramonium spp, belonging to the Solanaceae family. TAs’ dietary exposure can lead to harmful health effects, including anticholinergic toxicity, which can be life-threatening. This study aimed to find out the occurrence of TAs in cereal-based foods and evaluate the potential health risks associated with their consumption amongst children and adults in N’djamena, Chad. TAs were analyzed in 40 food samples collected from different households for the presence and levels of scopolamine and atropine using separate ELISA test kits. Findings showed that, the studied cereal-based food samples were contaminated with atropine and scopolamine at varied mean levels [millet (atropine: 2,417 µg/kg; scopolamine: 0.927 µg/kg), maize (atropine: 2,573 µg/kg; scopolamine: 0.779 µg/kg), and pearl millet (atropine: 2,515 µg/kg; scopolamine: 0.854 µg/kg)] with mean atropine levels exceeding the EU’s maximum limit (ML) of 1 µg/kg; as opposed to 25% (10/40) samples with mean scopolamine levels exceeding the EU’s maximum limit of 1 µg/kg. These f indings indicate a potential health risk for consumers: children and adults who regularly consume these cereal-based foods in N’djamena, Chad. Considering the estimated daily intake for children (0.07 µg/kg/day) and adults (0.132 µg/kg/day), and a Benchmark Dose Level (BMDL) at 1.54 µg/kg, the calculated Margin of Exposure (MOE) values were 22 for children and 11.66 for adults, implying the toxic contaminant is a public health concern, thus exposes consumer populations to associated health risks.
Keywords
• Tropane Alkaloids
• Dietary Exposure
• Risk Assessment
• Health Risks
• Adults
• Children
• Chad
Citation
Ambadi WI, Nfombouot HPN, Djomptchouang HT, Ali KH, Abia WA (2025) Occurrence and Health Risk Assessment of Tropane Alkaloids (Atropine and Scopolamine) in Cereal-Based Foods Consumed by Children and Adults in N’djamena, Chad. Ann Food Process Preserv 8(1): 1041.
INTRODUCTION
Tropane Alkaloids (TAs) produced as secondary plant metabolites are considered toxic and originate from various plant families, including Solanaceae, Brassicaceae, Erythroxylaceae, Euphorbiaceae, and Convulvulaceae [1]. Plants like D. stramonium are toxic due to the presence of TAswhich are found in various parts of the plants and can cause anticholinergic toxicity [2]. TAs found in various plant species serve as natural toxins that help plants adapt to stressful conditions [3]. Atropine and scopolamine are the two main TAs species found in TA-producing plants. They have been identified among over 200 tropane alkaloids (TAs) through their distinct chemical mechanism, which involves the esterification of a tropan-3α-ol backbone with tropic acid [4]. Atropine and scopolamine are rapidly and extensively absorbed from the gastrointestinal tract, followed by widespread distribution into tissues, and are primarily excreted in the urine [5]. Atropine and scopolamine have similar effects on the nervous system, where they act as anticholinergics, blocking the activity of acetylcholine, a neurotransmitter involved in neurotransmission [6]. They can induce a variety of acute effects, including: dilated pupils, change of heart rate, dryness of the mouth, constipation, urinary retention, and flushed skin [7]. Symptoms of acute toxicity typically manifest within 30 to 60 minutes following ingestion. The chemicals are typically excreted from the body and, as a result, they usually do not cause long-term health effects [8].The toxicological profile of tropane alkaloids is dominated by atropine and scopolamine, which are therefore the sole focus of this hazard characterization and exposure assessment [9]. Cereals like millet, pearl millet, and maize are vulnerable to contamination by TAs [10]. The presence of these compounds in cereal products suggests that plants like Datura stramonium may be present in the agricultural fields where these cereals are cultivated. These wild plants, typically found in cereal farms throughout the year, can grow between 50 and 100cm in height. They have dark green leaves of varying sizes and produce solitary, white flowers [11]. The flowers, in turn, yield capsule-shaped fruits that contain small, dispersed black and brown seeds [12]. These toxic seeds can be inadvertently harvested alongside food crops, particularly when the crops are of similar size, making it challenging to distinguish Datura stramonium seeds from those of cereal crops [7-13]. D. stramonium is commonly found in hot and tropical regions [14] Although some contamination reports have been attributed to the presence of seeds, other poisoning incidents have been linked to various plant parts, including leaves, flowers, stems, and roots [15]. Food and feed products are commonly contaminated with plant species like Atropa beladonna, Datura stramonium, and Hyoscyamus niger, which belong to the Solanaceae family [16]. The invasive nature of these plants in crops can lead to unintentional consumption of weed through accidental blending, posing a significant health risk to consumers [17]. Ingestion of contaminated food can cause serious adverse effects, including toxicity, hallucinations, and other harmful health outcomes [18]. vertently harvested alongside Datura stramonium weeds, leading to accidental contamination. Poisoning incidents related to the presence of these contaminants in various food commodities have been reported across several countries. For instance, Buckwheat contamination in France [19,20]. In Australia and France, canned beans and millet carrot balls were found to be contaminated [21,22]. Additionally, in Tanzania, wheat bread was also discovered to be contaminated, as reported in Van Meurs et al., [23]. Furthermore, many food contamination incidents have involved different plant parts, such as the leaves, as seen in cases of wild spinach and tea preparations collected from contaminated sources [16]. According to the European Food Safety Authority (EFSA) established in 2013, a group Acute Reference Dose (ARfD) of 0.016 μg/kg body weight (bw) was established, expressed as the sum of the chemicals. An ARfD is an estimate of the amount of a substance in food (and/or drinking-water), normally expressed on a body-weight basis, that can be ingested in 24 hours or less without appreciable health risk to the consumer based on all known facts at the time of evaluation [24]. The levels of atropine and scopolamine in baby food and processed cereal-based food for infants, young children, and adults containing millet, sorghum, buckwheat, maize, or their derived products should be regulated. A reference dose of 1µg/kg for atropine and 1µg/kg for Scopolamine is recommended [25]. The FAO/WHO concluded that establishing an ARfD was not possible and instead they selected a point of departure of 1.54 μg/kg bw for the sum of the two substances, based on decreased salivary secretion, and applied a margin of exposure approach [26]. However, comprehensive data on dietary exposure, occurrence of TAs in cereal foods, and associated health risks are limited. This study aimed to determine the levels of TAs (Atropine and Scopolamine) in cereal-based foods and to assess the dietary exposure levels and margin of exposure (MOE) for both children and adult consumer populations in N’Djamena, Chad.
MATERIALS AND METHODS
Sample collection
Cereal-based food samples were collected from different households randomly selected in the city of N’Djamena. A total of 40 food samples (processed cereal samples) were collected from 4 major neighborhoods, mainly Farcha, Gassi, Chadara talata, and Ndjare. Where cereals are highly consumed. The samples collected contain 3 types of processed cereal-based foods, namely millet (Panicum miliaceum), maize (Zea mays), and pearl millet (Pennisetum glaucum). Approximately 100-150 g of each sample was collected per home, placed in plastic zip-lock bags, stored at 4oC and directly transported to the laboratory of pharmacology and toxicology at the University of Yaounde I for atropine and scopolamine investigation.
Chemicals and reagents
Atropine and scopolamine standards were provided by ELISA kits purchased from Shanghai Ideal Medical Technology Co., Ltd (Shanghai, China, 2023). The atropine and scopolamine kit packages contain the necessary reagents for ELISA, like: sample diluents (phosphate- buffered saline (PBS)), ELISA enzymes (horseradish peroxidase (HRP)), chromogenic substrates (TMB (3,3,5,5-tetramethylbenzidine)), and stop solutions (sulfuric acid (H2SO4)). Methanol 70% was purchased from CODIMED company in Yaounde (Yaounde, Cameroon) for atropine extraction. Reagent like n-butanol was obtained from Douala market (Douala, Cameroon) for scopolamine extraction; hence, distilled water was purchased from the laboratory of organic chemistry of the University of Yaounde I.
Sample preparation
Atropine sample preparation: The sample for atropine determination was prepared using the method provided by the kit protocol described by Shanghai Ideal Medical Technology Co., Ltd (2023) without any modification. After milling, a 10 g dry sample was placed in a 50 ml conical tube where 20ml of 70% methanol was added. The solution underwent vigorous oscillation for approximately 3 minutes using a Vortexer. Then, the mixture was centrifuged (5804R, Eppendorf, Hamburg, Germany) at 6000rpm/min for 7 minutes at 4°C. The supernatant, containing the desired components extracted from the sample, was carefully separated and retained for further analysis or processing.
Scopolamine sample preparation: As proposed in the kit protocol Shanghai Ideal Medical Technology Co., Ltd (2023), scopolamine extraction involved preparing a solution of n-butanol: methanol: distilled water, respectively, in a volume ratio of 5:25:70 (V:V: V). Then, 9 ml of the solution was taken and added to the tube containing 1g of the sample. The mixture was then centrifuged (5804R, Eppendorf, Hamburg, Germany) at 4000 rpm/ min for 4 minutes. The centrifugation at high speed separated the solid particles from the liquid components (supernatants). The supernatant was carefully pipetted for further analysis.
TA Determination using Enzyme-Linked Immuno Sorbent Assay (ELISA)
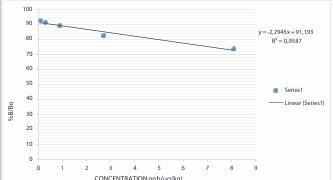
The ELISA method was employed to detect TA (atropine and scopolamine) in cereal-based food samples. A total of 40 samples were analysed using the ELISA assay. Two (2) ELISA kits were utilized separately for atropine and scopolamine. The Atropine ELISA kit was competitively sensitive. The competition occurred between the free atropine present in the samples and the pre-coated atropine-conjugated antigen on strips, which competed against atropine antibody conjugates for binding. In the case of the scopolamine ELISA kit, the wells of the microplate were coated with purified scopolamine antibodies, allowing them to adhere to the plate’s surface. Then, the sample, containing scopolamine, was added to the wells along with an antibody labeled with horseradish peroxidase (HRP) and scopolamine. During the incubation period, the scopolamine in the sample competed with the labeled antibody for the binding site on the immobilized antibodies in the wells. After reading the optical densities (OD) using a microplate reader at 450nm, both calibration curves for atropine (Figure 1) and scopolamine (Figure 2) were generated by plotting the real optical densities of different standards as a function of their respective concentrations.
Figure 1: Atropine calibration line (µg/kg).
Method Validation
The immunoassay analytical methods for toxic alkaloids, such as ELISA (Enzyme Linked Immuno-Sorbent Assay), were validated according to the guidelines of the Multidisciplinary Digital Publishing Institute(MDPI) [27]. Nevertheless, immunoassays likely have limitations and prospects compared to other analytical methods such as UPLC–MS/MS. The ELISA method offers several advantages: being affordable, rapid, easy, and sensitive with a considerable Limit of Quantification (LOQ), which is necessary for investigating toxic alkaloids, including Tropane Alkaloids (atropine and scopolamine), to ensure the safety of cereal-based foods Table 1.
Table 1. The reported immunoassays (ELISA) of toxic alkaloids [27].
|
Target |
Method |
Sample |
LOD |
Reference |
|
Scopolamine |
ELISA |
Hairy root cultures of a Duboisia hybrid. |
0.2 ng/mL |
[28] |
|
Atropine |
ELISA |
Pig urine, pork, and cereal flour. |
0.18 ng/mL |
[29] |
|
Benzoylhypaconine |
-------- |
Rat serum |
0.35 ng/mL |
[30] |
|
Diester alkaloids |
-------- |
Aconitum carmichaeli Debx. |
250 pg/mL |
[31] |
|
Aco-type alkaloids |
-------- |
Aconiti radixes |
100 ng/L |
[32] |
|
|
Banknotes. |
5.6 ng/L |
[33] |
|
|
Camptothecin |
-------- |
--- |
0.39 ng/mL |
[34] |
|
Morphine |
-------- |
Urine |
1.2 × 10−11 M |
[35] |
|
-------- |
Saliva |
6 ng/mL |
[36] |
|
|
Morphine-3- glucuronide |
-------- |
Urine |
762 pg/mL |
[35] |
|
Berberine |
-------- |
Plant |
780 ng/mL |
[37] |
|
Retrorsine |
-------- |
--- |
0.5 μg/mL |
[38] |
|
Triptolide |
-------- |
--- |
5 fM |
[39] |
LOD: the limit of detection; ELISA: Enzyme-Linked Immuno-Sorbent Assay
Dietary exposure estimation
The dietary exposure to TAs through millet consumption was obtained by calculating the Estimated Daily Intake (EDI) by focusing on the Daily Food Intake (DFI) for the population and the total mean concentration of both atropine and scopolamine (EFSA, 2023). Data on millet consumption were obtained from a survey via questionnaires administered in the field in N’Djamena, Chad. The Estimated Daily Intake (EDI) was calculated both for children and adults by using the following formula:
EDI = C x DFI
Where C was the total mean concentration of atropine or scopolamine in millet samples.
Acute health risk assessment
The Margin of Exposure (MOE) method was used to assess the health risk of TA exposure via millet-based food consumption. The Margin of Exposure (MOE) serves as a valuable tool for risk assessors when evaluating potential safety issues associated with the presence of substances in food and feed (EFSA, 2023). The MOE values were calculated combining the Estimated Daily Intake (EDI) and Benchmark dose level (BMDL), which is the dose at which a low but measurable adverse effect is observed.
|
|
After calculation, if the calculated MOE value is less than 100 (MOE<100), it indicates a potential public health concern associated with the presence of a toxic compound in the cereal-based foods. A MOE value greater than 100 (MOE>100) suggests that there is no significant public health concern regarding the toxic compound in the cereals.
Statistical analysis
This is an integral part of the research process, which ensures that data are properly organized, described, and preserved. In particular, Microsoft Excel 2016 was utilized to analyze data and generate frequency, distribution tables, and bar charts as means of presenting data. The data were summarized, analyzed, and interpreted to achieve the research objectives.
RESULTS AND DISCUSSION
Method validation
Atropine and scopolamine levels were measured in samples of various cereal types, including millet-based foods, maize-based foods, and pearl millet-based foods, using Enzyme-Linked Immunosorbent Assay (ELISA). This is a validated analytical method that can detect toxic alkaloids, as mentioned in the article. This analytical method has a considerable Limit of Quantification (LOQ) for atropine and scopolamine, which is 0.18 ng/mL [29] and 0.2 ng/mL [28], respectively.
Occurrence of TA in raw and processed cereals
Table 2 provides data on the levels of atropine and scopolamine across different cereal products. A total of 40 samples were collected, comprising 10 millet samples, 15 maize samples, and 15 pearl millet samples. The regulatory limit for atropine and scopolamine is 1 µg/kg, and experts recommend that the combined sum of atropine and scopolamine should not exceed 2µg/ kg (European Commission, 2023). Analysis of these samples revealed widespread contamination with tropane alkaloids, specifically atropine and scopolamine, exceeding regulatory limits in many samples. The results for scopolamine showed that 10 samples had high concentrations exceeding the regulatory limit (1µg/kg). In addition to these, 23 samples had detectable levels of scopolamine, but at concentrations below the regulatory limit (1µg/kg). Conversely, 07 samples had undetectable levels of scopolamine. Atropine, on the other hand, was found to be ubiquitous, present in all samples with very high concentrations far exceeding the regulatory limit (1µg/kg), with a minimum concentration of 1.95 µg/ kg, and a maximum concentration of 2.95µg/kg. These findings highlight the need for further examination and management strategies to address the presence of these contaminants in cereal products.
Table 2. Mean levels of TAs (atropine and scopolamine) in cereal-based food samples collected from participants’ households
|
Cereal products |
Number of samples N=40) |
Mean Levels of Atropine (µg/Kg) |
Mean Levels of Scopolamine (µg/Kg) |
Sum of atropine and scopolamine (µg/Kg) |
Sum of atro and scopo (µg/kg)> regulatory limit (2µg/kg) (EFSA Panel on Contaminants in the Food Chain (CONTAM), 2013) |
|
|
S1 |
2,125 |
0,959 |
3,084 |
+ |
|
|
S2 |
2,665 |
0,464 |
3,129 |
+ |
|
|
S3 |
2,026 |
0,383 |
2,409 |
+ |
|
|
S4 |
2,572 |
1,643 |
4,215 |
+ |
|
|
S5 |
2,731 |
0,374 |
3,105 |
+ |
|
|
S6 |
1,959 |
1,472 |
3,431 |
+ |
|
millet (n=10) |
S7 |
2,711 |
0,995 |
3,706 |
+ |
|
S8 |
2,715 |
ND |
2,715 |
|
|
|
|
S9 |
2,463 |
1,535 |
3,998 |
+ |
|
|
S10 |
2,204 |
0,518 |
2,722 |
+ |
|
Total mean (min-max) of TAs level µg/Kg |
2,417 (1,959-2,731) |
0,927 (0,374-1,643) |
3,251 (2,409-4,215) |
|
|
|
Total number (%) |
|
|
|
9 (22, 5%) |
|
|
|
S11 |
2,148 |
1,949 |
4,097 |
+ |
|
|
S12 |
2,213 |
0,095 |
2,308 |
+ |
|
|
S13 |
2,663 |
ND |
2,663 |
|
|
|
S14 |
2,669 |
1,283 |
3,952 |
+ |
|
|
S15 |
2,152 |
0,05 |
2,202 |
+ |
|
|
S16 |
2,503 |
0,437 |
2,94 |
+ |
|
|
S17 |
2,656 |
ND |
2,656 |
|
|
|
S18 |
2,792 |
ND |
2,792 |
|
|
|
S19 |
2,694 |
1,517 |
4,211 |
+ |
|
maize (n=15) |
S20 |
2,665 |
0,797 |
3,462 |
+ |
|
S21 |
2,342 |
ND |
2,342 |
|
|
|
|
S22 |
2,769 |
ND |
2,769 |
|
|
|
S23 |
2,511 |
0,617 |
3,128 |
+ |
|
|
|
|
|
|
|
|
|
S24 |
2,951 |
0,887 |
3,838 |
+ |
|
|
S25 |
2,875 |
0,167 |
3,042 |
+ |
|
Total mean (min-max) of TAs level µg/Kg |
2,573 (2,148-2,951) |
0,779 (0,05-1,949) |
3,093 (2,202-4,211) |
|
|
|
Total number (%) |
|
|
|
10(25%) |
|
|
|
S26 |
2,476 |
0,779 |
3,255 |
+ |
|
|
S27 |
2,542 |
0,590 |
3,132 |
+ |
|
|
S28 |
2,659 |
1,841 |
4,5 |
+ |
|
|
S29 |
2,542 |
0,842 |
3,384 |
+ |
|
|
S30 |
2,452 |
0,329 |
2,781 |
+ |
|
|
S31 |
2,530 |
1,508 |
4,038 |
+ |
|
|
S32 |
2,359 |
0,500 |
2,859 |
+ |
|
|
S33 |
2,577 |
1,742 |
4,319 |
+ |
|
|
S34 |
2,941 |
0,392 |
3,333 |
+ |
|
|
S35 |
2,053 |
0,545 |
2,598 |
+ |
|
Pearl millet (n=15) |
S36 |
2,704 |
1,778 |
4,482 |
+ |
|
S37 |
2,259 |
ND |
2,259 |
|
|
|
S38 |
2,445 |
0,689 |
3,134 |
+ |
|
|
|
S39 |
2,577 |
0,401 |
2,978 |
+ |
|
|
S40 |
2,610 |
0,023 |
2,633 |
+ |
|
Total mean (min-max) of TAs level µg/Kg |
2,515 (2,053-2,941) |
0,854 (0,023-1,841) |
3,312 (2,259-4,5) |
|
|
|
Total number (%) |
|
|
|
14(35%) |
|
|
Total mean (range) of TAs level µg/kg |
2,5 (2,05-2,874) |
0,85 (0,33-1,811) |
3,22 (2,29-4,3) |
|
|
|
Total number (%) |
|
|
33 (82, 5%) |
33 (82, 5%) |
|
ND: Not Detectable, S: Sample
The presence of tropane alkaloids (atropine and scopolamine) contamination in most of the samples collected from the study households (N=40) is not surprising, given the high susceptibility of cereals to such contamination. This finding aligns with a report by Vukovi? et al. [15] in Serbia, where 31.1% of their tested samples (103 samples in total) were contaminated with atropine and scopolamine at concentrations above the limit of quantification (LOQ).
The mean (range) levels of tropane alkaloids in our samples were:
- Maize samples (n=15):2.573 (2.148-2.951) µg/ kg for atropine and 0.778 (0.05-1.949) µg/kg for scopolamine; with a mean (range) sum of 3.093 (2.202-4.211) µg/kg.
- Millet samples (n=10): 2.417 (1.959-2.731) µg/ kg for atropine and 0.927 (0.374- 1.643) µg/kg for scopolamine, with a mean (range) sum of 3.251 (2.409-4.215) µg/kg.
- In pearl millet samples (n=15): 2.515 (2.053-2.941) µg/kg for atropine and 0.854 (0.023-1.841) µg/kg for scopolamine, with a mean (range) sum of atropine and scopolamine of 3.312 (2.259-4.5) µg/kg.
- The overall mean (range) levels of TAs in all ‘cereal based foods’ samples (N=40) collected from our study participants were 2.515 (2.05-2.874) µg/kg for atropine and 0.85 (0.33-1.811) µg/kg for scopolamine, with a total mean (range) of atropine and scopolamine in all ‘cereal based foods’ of 3.22 (2.29-4.3) μg/kg.
The overall mean (range) levels of TAs in all ‘cereal based foods’ samples (N=40) collected from our study participants were 2.515 (2.05-2.874) µg/kg for atropine and 0.85 (0.33-1.811) µg/kg for scopolamine, with a total mean (range) of atropine and scopolamine in all ‘cereal based foods’ of 3.22 (2.29-4.3) μg/kg. These levels in our samples are higher than those reported by [40] in corn puff samples, where 22.2% of the samples tested positive for atropine (1.03-1.58 μg/kg) and scopolamine (0.29-0.47 μg/kg). However, the tropane alkaloid levels in our samples are lower than those revealed by [41,42] in their 12 popcorn samples, which tested positive for atropine (from 5.3 to 28.0 µg/kg) and scopolamine (from 2.1 to 6.3 µg/kg). Notably, the mean quantity of tropane alkaloids in our pearl millet samples (3.312 µg/kg) was higher than in millet (3.251 µg/kg) and maize samples (3.0312 µg/kg). In light of this, it is concerning to note that the quantity of atropine and scopolamine in most of our samples exceeded the regulatory limits (1 µg/kg for atropine, 1 µg/ kg for scopolamine, and 2 µg/kg for the sum of atropine and scopolamine) set by the European Commission [25]; indicating that our samples are highly contaminated with atropine and scopolamine and consumers are exposed to tropane alkaloids through their diets.
Daily dietary exposure estimation and health risk characterization
The average Estimated Daily Intake (EDI) of the selected cereals for adults and children was as follows:
- For Adults, the EDI values are 0.053 kg, 0.035 kg, and 0.035 kg for millet, maize, and pearl millet, respectively.
- For children, the EDI values are 0.025 kg, 0.017 kg, and 0.025 kg for millet, maize, and pearl millet, respectively.
The Margin of Exposure (MOE) for each cereal type was calculated using a safety threshold of 1.54 µg/kg [26]. Table 3 below presents the minimum and maximum values of dietary exposure and the corresponding MOE for each type of cereal. This information provides a clear understanding of the range of exposure and the margin of safety associated with the consumption of these cereals.
Table 3. Dietary exposure and margin of exposure of different cereal types
|
|
Children |
Adults |
||||||
|
Types of cereals (n=40) |
DE(µg/kg) |
MOE |
DE(µg/kg) |
MOE |
||||
|
|
Min |
Max |
Min |
Max |
Min |
Max |
Min |
Max |
|
Millet(n=10) |
0,06 |
0,105 |
25,66 |
14,66 |
0,127 |
0,223 |
12,12 |
6,9 |
|
Maize (n=15) |
0,037 |
0,071 |
41,62 |
21,69 |
0,077 |
0,147 |
20 |
10,47 |
|
Pearl millet (n=15) |
0,056 |
0,112 |
27,5 |
13,75 |
0,079 |
0,157 |
19,49 |
9,8 |
The estimated daily food intake for children is 0.022 kg/day, resulting in a dietary exposure (DE) of 0.07µg/ kg/day and a margin of exposure (MOE) of 22. For adults, the estimated daily intake is 0.041 kg/day, leading to a DE of 0.132 µg/kg/day and an MOE of 11.66. An MOE below 100 suggests a potential public health concern, as per the guidelines set by the European Food Safety Authority [25]. These findings indicate that the presence of these toxic compounds in cereal-based foods may pose a risk to consumers. For the three types of cereals analyzed (millet, maize, and pearl millet), different dietary exposure (DE) and margin of exposure (MOE) values were obtained. Specifically, millet consumption resulted in dietary exposure ranging from 0.06 to 0.105µg/kg for children and from 0.127 to 0.223µg/ kg for adults; the corresponding MOE ranges from 25.66 to 14.66 for children and from 12.12 to 6.9 for adults. Maize consumption resulted in dietary exposure (DE) ranging from 0.037 to 0.071µg/kg for children and from 0.077 to 0.147µg/kg for adults, with the corresponding MOE ranging from 41.62 to 21.69 for children and from 20 to 10.47 for adults. Pearl millet consumption, on the other hand, resulted in dietary exposure ranging from 0.056 to 0.112µg/kg for children and from 0.079 to 0.157µg/kg for adults, and the corresponding MOE ranged from 27.5 to 13.75 for children and from 19.49 to 9.8 for adults. The corresponding margin of exposure values for all three cereal types were below the recommended level of 100, suggesting that both children and adults were at risk of adverse health effects associated with the consumption of these cereals. The margin of exposure (MOE) for children was determined to be 22, while for adults it was 11.66. The study revealed that both children and adults have margin of exposure (MOE) values below the threshold of 100, indicating a potential health concern associated with tropane alkaloid exposure. Furthermore, it is essential to consider that the study population’s consumption of other food items, such as groundnut-based foods, fruits (such as okra and sorrel), cassava leaves, potatoes, green tea, and cereal-based foods like cereals, donuts, and bowl cereals, may exacerbate the margin of exposure values. These additional dietary sources can contribute to an increased intake of tropane alkaloids. This observation aligns with a study conducted in Uganda by Abia et al. [11], which also reported the presence of tropane alkaloids in various food items. Considering these findings, it is crucial to address the contamination of tropane alkaloids in a wider range of food products to ensure the safety and well-being of the population.
CONCLUSION
In conclusion, this pioneering study in Chad investigated the occurrence and health risk assessment of tropane alkaloids in cereal-based foods consumed by children and adults in Central Africa. We calculated the estimated daily intake by combining daily food intake data with the total concentrations of tropane alkaloids detected in positive samples. This approach enabled us to accurately assess dietary exposure. Our findings revealed that current levels of tropane alkaloids, atropine and scopolamine, exposure from cereal-based foods are likely to have adverse health effects on children and adults. Given the low awareness levels regarding tropane alkaloids and food contaminants in general, educational interventions are necessary. Our study highlights the need for continued monitoring and risk assessment of tropane alkaloids in cereal-based foods. While we contributed valuable data on dietary exposure, limitations included the use of analytical methods with limited sensitivity and a restricted geographic scope. Future research should prioritize developing more sensitive analytical methods, expanding investigations to other regions, and exploring the occurrence of tropane alkaloids in a wider range of food products. Additionally, studies should investigate the potential health effects of chronic exposure to tropane alkaloids and explore strategies to reduce this exposure. Ultimately, our study emphasizes the need for enhanced awareness, education, and regulation to mitigate the risks associated with tropane alkaloid exposure in cereal-based foods.
ACKNOWLEDGMENTS
This study was self-funded and scientifically supervised by Dr. Wilfred Angie Abia in the laboratory of pharmacology and toxicology of Pr. Moundipa Paul Fewou of the University of Yaounde, Cameroon.
REFERENCES
- Griffin WJ, Lin GD. Chemotaxonomy and geographical distribution of tropane alkaloids. Phytochemistry. 2000; 53: 623-637.
- Disel NR, Yilmaz M, Kekec Z, Karanlik M. Poisoned after Dinner: Dolma with Datura Stramonium. Turk J Emerg Med. 2016; 15: 51-55.
- Boualem Harfi, Lakhdar Khelifi, Majda Khelifi-Slaoui, Corinne Assaf- Ducrocq, Eric Gontier. Tropane alkaloids GC/MS analysis and low dose elicitors’ effects on hyoscyamine biosynthetic pathway in hairy roots of Algerian Datura species. Scientific Reports volume. 2018; 8: 17951.
- Han S, Jang S, Oh S, Lee J, Lee HJ, Koo YE, et al. Occurrence and health risk assessment of tropane alkaloids in cereal foods consumed in Korea. Food Chem Toxicol. 2024;186: 114589.
- Kohnen-Johannsen KL, Kayser O. Tropane Alkaloids: Chemistry, Pharmacology, Biosynthesis and Production. Molecules. 2019; 24: 796.
- Lochner M, Thompson AJ. The muscarinic antagonists scopolamine and atropine are competitive antagonists at 5-HT3 receptors. Neuropharmacology. 2016;108: 220-228.
- Perhari? L, Juvan KA, Stanovnik L. Acute effects of a low-dose atropine/scopolamine mixture as a food contaminant in human volunteers. J Appl Toxicol. 2013; 33: 980-990.
- National Research Council (US) Panel on Anticholinesterase Chemicals, National Research Council (US) Panel on Anticholinergic Chemicals, 1982. Possible Long-Term Health Effects of Short- Term Exposure to Chemical Agents: Volume 1 Anticholinesterases and Anticholinergics. National Academies Press (US), Washington (DC).
- European Food Safety Authority (EFSA); Arcella D, Altieri A, HorváthZ. Human acute exposure assessment to tropane alkaloids. EFSA J.2018; 16: e05160.
- González-Gómez L, Morante-Zarcero S, Pérez-Quintanilla D, SierraI. Occurrence and Chemistry of Tropane Alkaloids in Foods, with a Focus on Sample Analysis Methods: A Review on Recent Trends and Technological Advances. Foods. 2022;11: 407.
- Abia WA, Montgomery H, Nugent AP, Elliott CT. Tropane alkaloid contamination of agricultural commodities and food products in relation to consumer health: Learnings from the 2019 Uganda food aid outbreak. Compr Rev Food Sci Food Saf. 2021; 20: 501-525.
- Jean-Pierre Goullé, Gilbert Pépin, Véronique Dumestre-Toulet, Christian Lacroix. Botanique, chimie et toxicologie des solanacées hallucinogènes: belladone, datura, jusquiame, mandragore. Annales de Toxicologie Analytique. EDP Sciences. 2014; 16: 22-35.
- Caligiani A, Palla G, Bonzanini F, Bianchi A, R. Bruni. A validated GC–MS method for the detection of tropane alkaloids in buckwheat (Fagopyron esculentum L.) fruits, flours and commercial foods. Food Chem. 2011; 127: 204-209.
- Moffat P. Setshogo. A review of some medicinal and or hallucinogenic Solanaceous plants of Botswana: the genus Datura L. Int J Appl Res Nat Prod. 2015; 1: 15-23.
- Vukovi? G, Stojanovi? T, Konstantinovi? B, Bursi? V, Puva?a N, PopovM, et al. Atropine and Scopolamine in Maize Products from the Retail Stores in the Republic of Serbia. Toxins (Basel). 2022; 14: 621.
- Patrick P.J. Mulder, Monique de Nijs, Massimo Castellari, Maria Hortos, Susan MacDonald, Colin Crews, Jana Hajslova, Milena Stranska. Occurrence of tropane alkaloids in food. EFSA Support. Publ. 2016; 13: 1140E
- P. Adamse, H.P. van Egmond, M.Y. Noordam, P.P. J. Mulder, M. de Nijs. Tropane alkaloids in food: poisoning incidents. Qual. Assur. Saf. Crops Foods. 2014; 6: 15-24.
- Koleva II, van Beek TA, Soffers AE, Dusemund B, Rietjens IM. Alkaloids in the human food chain--natural occurrence and possible adverse effects. Mol Nutr Food Res. 2012; 56: 30-52
- Cirlini M, Demuth TM, Biancardi A, Rychlik M, Dall’Asta C, Bruni R. Are tropane alkaloids present in organic foods? Detection of scopolamine and atropine in organic buckwheat (Fagopyron esculentum L.) products by UHPLC-MS/MS. Food Chem. 2018; 239: 141-147.
- Rodríguez Galván, M., 2018. Perfil fitoquímico de especies vegetales de Guanajuato y sus alrededores por cromatografía en capa fina de alta resolución (HPTLC) (Master’s Thesis). Tesis (MC)– Centro de Investigación y de Estudios Avanzados del IPN Unidad ….
- P. Adamse, H.P. van Egmond. 2010. Tropane alkaloids in food.
- Fretz R, Schmid D, Brueller W, Girsch L, Pichler AM, Riediger K, et al. Food poisoning due to Jimson weed mimicking Bacillus cereus food intoxication in Austria, 2006. Int J Infect Dis. 2007; 11: 557-558.
- van Meurs A, Cohen A, Edelbroek P. Atropine poisoning after eating chapattis contaminated with Datura stramonium (thorn apple). Trans R Soc Trop Med Hyg. 1992; 86: 221.
- European Food Safety Authority. EFSA explains risk assessment: glyphosate. Publications Office, LU. 2015.
- Margin of Exposure. EFSA [WWW Document]. 2023.
- European Food Safety Authority (EFSA), Binaglia M. Assessment of the Conclusions of the Joint FAO/WHO Expert Meeting on Tropane Alkaloids. EFSA J. 2022; 20: e07229;
- Ren Z, Zhang H, Wang Z, Chen X, Yang L, Jiang H. Progress in Immunoassays of Toxic Alkaloids in Plant-Derived Medicines: A Review. Toxins (Basel). 2022; 14: 165.
- Kikuchi Y, Irie M, Yoshimatsu K, Ishimaru K, Shimomura K, Satake M, et al. A monoclonal antibody to scopolamine and its use for competitive enzyme-linked immunosorbent assay. Phytochemistry. 1991; 30: 3273-3276
- Wang LM, Zhang Z, Yao RZ, Wang GL. The Role of Intrathecal Morphine for Postoperative Analgesia in Primary Total Joint Arthroplasty under Spinal Anesthesia: A Systematic Review and Meta-Analysis. Pain Med. 2021; 22: 1473-1484.
- Can-Can Liu, Yun-Hui Xu, Shuai Yuan, Yu Xu, Mo-Li Hua. An enzyme-linked immunosorbent assay for monoester-type aconitic alkaloids and its application in the pharmacokinetic study of benzoylhypaconine in rats. J. Asian Nat. Prod. Res. 2018; 20: 352-360.
- Xiangting Gao, Jun Hu, Xincai Zhang, Yuanyi Zuo, Yun Wang, Shaohua Zhu. Research progress of aconitine toxicity and forensic analysis of aconitine poisoning. Forensic Sci. Res. 2020; 5: 25-31.
- Kido K, Edakuni K, Morinaga O, Tanaka H, Shoyama Y. An enzyme- linked immunosorbent assay for aconitine-type alkaloids using an anti-aconitine monoclonal antibody. Anal Chim Acta. 2008; 616: 109-114.
- Abdelshafi NA, Panne U, Schneider RJ. Screening for cocaine on Euro banknotes by a highly sensitive enzyme immunoassay. Talanta. 2017; 165: 619-624.
- Yan L, Nan X, Zhang C, Wang H, Huang X, Hu J, et al. Development of an enzyme-linked immunosorbent assay for camptothecin. Mol Med Rep. 2019; 20: 959-966.
- Dillon PP, Daly SJ, Manning BM, O’Kennedy R. Immunoassay for the determination of morphine-3-glucuronide using a surface plasmon resonance-based biosensor. Biosens Bioelectron. 2003; 18: 217-227.
- Brennan J, Dillon P, O’Kennedy R. Production, purification and characterisation of genetically derived scFv and bifunctional antibody fragments capable of detecting illicit drug residues. J Chromatogr B Analyt Technol Biomed Life Sci. 2003; 786: 327-342.
- Jun-Sik Kim, Hiroyuki Tanakaa, Yukihiro Shoyama. Immunoquantitative analysis for berberine and its related compounds using monoclonal antibodies in herbal medicines. Analyst. 2004; 129: 87-91.
- Roseman D M, Wu, X Kurth M J. Enzyme-Linked Immunosorbent Assay Detection of Pyrrolizidine Alkaloids: Immunogens Based on Quaternary Pyrrolizidinium Salts. Bioconjug. Chem. 1996; 7: 187-195.
- Lapinjoki SP, Veräjänkorva HM, Huhtikangas AE, Lehtola TJ, Lounasmaa M. An enzyme-linked immunosorbent assay for the antineoplastic agent vincristine. J Immunoassay. 1986; 7: 113-128.
- Stojanovi? Tijana N, Vukovi? Gorica Lj, Petrovi? Aleksandra P, Konstantinovi? Bojan B, Puva?a Nikola M, Marinkovi? Dušan S, et al. Determination of tropane alkaloids in corn puffs by the LC-MS/MS. Zb. Matice Srp. Za Prir. Nauke. 2021;141: 69-80.
- Vukovi? G, Stojanovi? T, Konstantinovi? B, Bursi? V, Puva?a N, Popov M, et al. Atropine and Scopolamine in Maize Products from the Retail Stores in the Republic of Serbia. Toxins (Basel). 2022; 14: 621.
- EFSA Panel on Contaminants in the Food Chain (CONTAM). Scientific Opinion on Tropane alkaloids in food and feed. EFSA J. 2013; 11.