Prospective Multicenter Blinded Randomized Study Comparing PP and PVDF Mesh Implants in Lichtenstein Procedure with Respect to Pain and Recurrence
- 1. La Fe University Hospital, Spain
- 2. Hospital Nuestra Senora del Rosario, Spain
- 3. Complejo Hospitalario de Toledo, Spain
- 4. La Mancha-Centro General Hospital, Spain
- 5. Hospital Municipal de Badalona, Spain
- 6. Hospital Juan Ramon Jimenez, Spain
- 7. Henares University Hospital, Francisco de Vitoria University, Spain
- 8. Hospital Reina Sofia, Spain
- 9. University Hospital, Santiago de Compostela, Spain
- 10. University Hospital del Mar, Spain
- 11. Valld’HebrónUniversity Hospital, Spain
- 12. Fundacion Alcorcon University Hospital, Spain
- 13. Hospital de Torrejón de Ardoz, Spain
- 14. Hospital Santa Cristina, Spain
ABSTRACT
Introduction: In the past decades tension-free inguinal hernia repair using prosthetic meshes has become the procedure of choice. Recurrence rates could be reduced, but still remain one of the major complications. Another major problem is pain of which both acute and chronic pain can influence the patients’ quality of life directly.
Material and methods: We conducted a single blinded multicenter randomized trial with 164 patients treated for primary bilateral inguinal hernia with a total follow-up period of one year. The open tension-free Lichtenstein technique as described by Amid was used as standardized procedure. In each patient, we used two different meshes. The Surgipro® is composed of polypropylene (PP) and the DynaMesh®-LICHTENSTEIN of polyvinylidene fluoride (PVDF). This approach allowed us to investigate pain, chronic pain and other postoperative complications in dependence of the used mesh.
Results: Overall, both biomaterials performed well over the full follow-up period of one year, but the DynaMesh-LICHTENSTEIN was significantly better in terms of late postoperative pain and chronic pain at 3-months and 6-months follow-up compared to the Surgipro. Throughout the whole follow-up of one year, we recorded a single recurrence for the PVDF mesh and three recurrences for the PP mesh.
Conclusion: Data from this study suggests that the use of large pore PVDF meshes is preferable compared to PP small pore meshes because it significantly decreases pain and chronic pain up to 6 months after surgery and acute foreign body sensation. After one year, there are still differences in pain but these are no more significant.
KEYWORDS
Lichtenstein procedure; Bilateral inguinal hernia ; Recurrence; Pain; PVDF; Mesh implant.
CITATION
García-Pastor P, Hidalgo M, Gutierrez R, Picazo J, lopart RL, et al. (2018) Prospective Multicenter Blinded Randomized Study Comparing PP and PVDF Mesh Implants in Lichtenstein Procedure with Respect to Pain and Recurrence. JSM Surg Proced 1(1): 1002
ABBREVIATIONS
Polyvinylidene fluoride (PVDF); Polypropylene (PP)
INTRODUCTION
Inguinal hernia repair is one of the most performed surgical treatments with over 20 million patients annually worldwide [1]. For men and women the lifetime risk of inguinal hernia which needs treatment is estimated to be 27% and 3%, respectively[2]. Although surgical treatment is successful in the majority of cases recurrences which require reoperation still occur in 10-15% of cases. Beside recurrence, long-term disability due to pain is a problem and occurs in 10-12% of cases [1]. Thus, both recurrence and pain are still major complications in inguinal hernia surgery. There are several risk factors linked to chronic pain such as the intraoperative nerve management, the use of different fixation methods, the mesh material or its pore size although this is an ongoing debate in literature [3-5]. Today, the Lichtenstein technique, which was introduced in 1986 and named after its inventor, is the standard open tension-free method for inguinal hernia repair and often referred to as the “gold standard”[6]. In order to minimize chronic pain after inguinal hernia repair, one of the key factors is to identify the “best” mesh composed of the “best” biomaterial. Both Polypropylen (PP) and Polyvinylidene fluoride (PVDF) are well-established and widely used polymer for medical devices. However, PVDF seems to provide some crucial benefits as its high biocompatibility, long-term stability and the unnecessity of additional additives might increase its clinical impact in the future [1,7-13].
Objective
The objective of this study was to compare the performance of a PVDF- and a PP-mesh in terms of pain, chronic pain, comfort (numbness and foreign body sensation) and postoperative complications after primary bilateral inguinal hernia repair during a follow-up of one year
MATERIALS AND METHODS
Study design and setting
The study is a prospective multicenter blinded randomized trial. Overall 19 Spanish centers participated in the study. The majority of cases (65%) were included by five centers. Patients were operated either as out-patient without hospitalization or inpatient. All operations were performed by surgeons experienced in abdominal wall surgery (only consultants, no residents).
Participants - Inclusion criteria
Only male patients with primary bilateral inguinal hernia (Aachener classification LI-III, MI-III, McI-III[14]), aged between 17 and 90 were included in the study. All patients were asked to sign the written informed consent prior to the intervention and only those who agreed were included into the study.
Participants - Exclusion criteria
Our exclusion criteria were defined as: ASA score IV and higher, emergency surgery, recurrent hernia, scrotal hernia, coagulation disorders, neurological disorders, psychologically instable and refusal to sign informed consent.
Outcome parameters
Pain and chronic pain were defined as primary outcome parameters. Additional patient reported outcome parameters were foreign body sensation and numbness. As secondary outcome parameters the early (≤ 7 days post-operatively: seroma, hematoma, infection, early recurrence) and late (> 7 days ≤ 1 year post-operatively: recurrence, mesh migration) postoperative complications were recorded.
Surgical technique
All patients were operated either under general, spinal or local anesthesia with the Lichtenstein technique standardized according to Amid described in [6]. Amid emphasized the importance of standardization of the procedure however at the same time he endorsed technical considerations whenever necessary. These technical considerations include nerve and/or hernia sac resection. All patients were treated with a Polypropylen (PP) mesh on one side and a Polyvinyliden fluoride (PVDF) mesh on the other side. As PP meshSurgipro® by Covidien/Medtronic, Mansfield, USA was used. Surgipro is a flat mesh made from PP monofilaments (Ø ~ 140 µm) with a textile porosity of 49%. As PVDF mesh DynaMesh®- LICHTENSTEIN by FEG TextiltechnikmbH, Aachen, Germany was used. DynaMesh-LICHTENSTEIN is a flat but pre-slit mesh made from PVDF monofilaments (Ø ~ 130 µm) with a textile porosity of 73%. Patients were not aware of which side the PP or PVDF mesh was placed. The blinded study design ensured that the assessment of the patient reported outcome (PRO) parameters was independent of the mesh type. For the fixation of the mesh to the inguinal ligament Prolene 00 (Ethicon, Johnson and Johnson Company, Cincinnati, OH, USA) was used and the oblique muscle - tendon assembly was perfomed using Vicryl 00 (Ethicon, Johnson and Johnson Company, Cincinnati, OH, USA).
Follow-up
Patients were invited to an outpatient clinical follow up at the 7th day, 3 months, 6 months and 12 months postoperatively. The clinical follow up was not necessarily done by the same surgeon who performed the intervention. Patients were asked about their pain perception using the visual analogue scale (VAS). Patients with a VAS score > 4 at the 3-months follow-up or later were considered as chronic pain patients. In addition the patients were asked to assess the PRO parameters “foreign body sensation” and “numbness”, which were defined as dichotomous variables.
Study design limitations
(1) A limitation of the study design is the single blinded design. In a double blinded design the surgeon performing the clinical follow-up would not have been aware of the mesh type used on each side. (2) Further limitation of the study design might be the capturing of PRO data by simple dichotomous categorical variables or VAS scales instead of comprehensive standardized questionnaires as CPPS (chronic pain prevention screener), SPS (surgical pain scale), IPQ (inguinal pain scale) or EQ-5D. With each of these standardized questionnaires we would have substantially extended the case report form (CRF). Considering the situation of a financially underpowered study we faced the dilemma of having either a comprehensive CRF and just a low number of FUs, or a lean CRF and in return a higher number of FUs. Finally, we decided upon the lean CRF and the higher number of FUs, in the knowledge that this is a trade-off decision which has its limitation. (3) It is known that a considerable number of recurrences occur much later than 1 year [15]. (4) Preoperative pain (VAS > 0) in the groin was assessed by the patients using the VAS scale. However, the reason for pain was not further examined. Preoperative pain in the groin which was not linked to the hernia might bias the postoperative pain assessment and limit the interpretation of its temporal course.
Statistical methods
The statistical methodology was chosen and performed by an independent statistician. Data was analysed with SPSS (SPSS for Windows, Chicago: SPSS Inc.). For categorical variables the Chi Square test of independence was used while the Student’s t-test was used for continuous variables. Statistical significance was assumed at p-value < 0.05
RESULTS
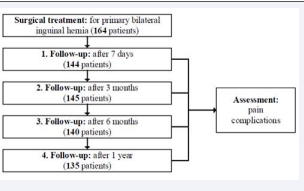
A total of 164 male patients were included in the study. 135 patients participated throughout the whole follow-up of one-year resulting in a dropout rate of 17.7% (Figure 1).
Figure 1 Study flow-chart
Patient characteristics (demographics, patient variables as ASA classification and risk factors, type of hernia) and operative date (anaesthesia, duration of surgery, resection of nerves and hernia sac, antibiotic prophylaxis) are listed in Table 1.
Table 1: Patient characteristics and operative data.
|
Table 1: Patient characteristics and operative data. |
||
|
|
n/N or mean |
range or % |
|
Patient demographics |
|
|
|
male gender |
164/164 |
|
|
age (mean years) |
61.5 |
33-86 |
|
Patient variables |
|
|
|
ASA I |
36/164 |
22 |
|
ASA II |
100/164 |
61 |
|
ASA III |
28/164 |
17 |
|
Obesity |
39/164 |
24 |
|
Smoker |
44/164 |
27 |
|
COPD |
29/164 |
18 |
|
Hernia variables |
|
|
|
(PP-side -- PVDF-side) |
|
|
|
LI |
7 -- 5/164 |
4.3 -- 3.0 |
|
LII |
45 -- 48/164 |
27.4 -- 29.3 |
|
LIII |
12 -- 12/164 |
7.3 -- 7.3 |
|
MI |
9 -- 8/164 |
5.5 -- 4.9 |
|
MII |
64 -- 52/164 |
39.0 -- 31.7 |
|
MIII |
15 -- 22/164 |
9.1 -- 13.4 |
|
McI |
2 -- 4/164 |
1.2 -- 2.4 |
|
McII |
8 -- 10/164 |
4.9 -- 6.1 |
|
McIII |
2 -- 3/164 |
1.2 -- 1.8 |
|
Operative setting |
|
|
|
in-patient |
108/164 |
65.9 |
|
out-patient |
56/164 |
34.1 |
|
Anesthesia |
|
|
|
local |
5/164 |
3 |
|
spinal |
116/164 |
70.7 |
|
general |
43/164 |
26.2 |
|
Operative variables |
|
|
|
(PP-side -- PVDF-side -- bilateral) |
|
|
|
duration of surgery (min) |
30.2 -- 29.9 |
15-55 -- 15-55 |
|
resection hernia sac |
12 -- 3 -- 15/164 |
7.3 -- 1.8 -- 9.1 |
|
resection N. Ilioinguinalis |
3 -- 3 -- 16/164 |
1.8 -- 1.8 -- 9.8 |
|
resection N. Hypogastricus |
3 -- 3 -- 8/164 |
1.8 -- 1.8 -- 4.9 |
|
resection N. Genitofemoralis |
4 -- 9 -- 3/164 |
2.4 -- 5.5 -- 1.8 |
|
antibiotic prophylaxis |
164/164 |
100 |
|
trombosis prophylaxis |
82/164 |
50 |
It is to note that 61% of the patients were classified as ASA II and that 65.9% of the patients were treated as inpatients.
Table 2 lists early postoperative complications within the first 7 days after surgery. At this time point, comparing both sides only the difference of the foreign body sensation was statistically significant favouring the PVDF-side (p < 0.001). The number of recorded seromas and haematomas was lower for the PVDF-side, but not significant.
|
Table 2: Early postoperative complications (follow-up: 7th day). |
||||
|
PP-side |
PVDF-side |
p-value |
bilateral |
|
|
seroma |
7 (4.9%) |
3 (2.1%) |
0.198 |
2 (1.4%) |
|
haematoma |
11 (7.6%) |
5 (3.5%) |
0.123 |
62 (43.1%) |
|
infection |
0 (0.0%) |
1 (0.7%) |
- |
0 (0.0%) |
|
early recurrence |
0 (0.0%) |
0 (0.0%) |
- |
0 (0.0%) |
|
foreign body sensation |
32 (22.2%) |
8 (5.6%) |
<0.001 |
38 (26.4%) |
|
numbness |
36 (25.0%) |
31 (21.5%) |
0.486 |
29 (20.1%) |
Table 3 shows late postoperative complications including chronic pain recorded at the 3 months, 6 months and 1 year follow-up. Over the full time period of one year, three recurrences on the PP-side and one on the PVDF-side were recorded. There was no case of mesh migration for both sides. At the 3 months (p = 0.001) and 6 months (p = 0.05) follow-up, there were significantly less patients with chronic pain on the PVDF-side. Both sides had only one patient with chronic pain after one year.
|
Table 3: Late postoperative complications including chronic pain. |
|||||
|
follow-up |
|
PP-side |
PVDF-side |
p-value |
bilateral |
|
3 months |
recurrence |
1 (0.7%) |
0 (0.0%) |
- |
0 (0.0%) |
|
|
mesh migration |
0 (0.0%) |
0 (0.0%) |
- |
0 (0.0%) |
|
|
chronic pain VAS>4 |
15 (10.3%) |
2 (1.4%) |
0.001 |
5 (3.4%) |
|
|
numbness |
5 (3.4%) |
12 (8.3%) |
0.08 |
22 (15.2%) |
|
6 months |
recurrence |
1 (0.7%) |
1 (0.7%) |
- |
0 (0.0%) |
|
|
mesh migration |
0 (0.0%) |
0 (0.0%) |
- |
0 (0.0%) |
|
|
chronic pain VAS>4 |
8 (5.7%) |
2 (1.4%) |
0.05 |
2 (1.4%) |
|
|
numbness |
6 (4.3%) |
3 (2.1%) |
0.25 |
4 (2.9%) |
|
1 year |
recurrence |
1 (0.7%) |
0 (0.0%) |
- |
0 (0.0%) |
|
|
mesh migration |
0 (0.0%) |
0 (0.0%) |
- |
0 (0.0%) |
|
|
chronic pain VAS>4 |
1 (0.7%) |
1 (0.7%) |
0.751 |
2 (1.5%) |
|
|
numbness |
2 (1.5%) |
2 (1.5%) |
0.689 |
3 (2.2%) |
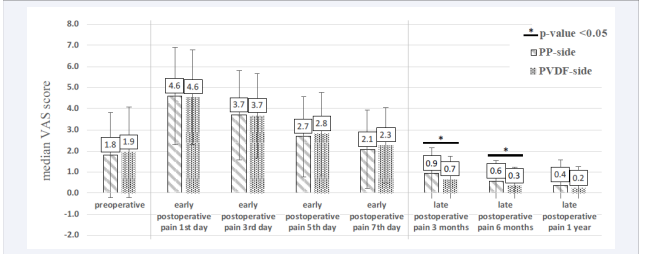
Pain assessment was conducted with a VAS scale as stated above. It was recorded preoperatively and postoperative on the 1st, 3rd, 5th and 7th day as well as the 3rd and 6th month and after one year. The median VAS scores and their standard deviations are shown in Figure 2.
Figure 2 Preoperative, early postoperative (≤ 7days), and late postoperative (7 > days ≤ 1year) pain assessment via visual analogue scale (VAS). Bars display median VAS scores and standard deviations for each mesh type at the follow-ups. Adjacent bars with a horizontal bar and an asterisk above mark timepoints at which the difference between the two mesh types was statistically significant (p-value < 0.05).
The preoperative median VAS scores were 1.8 and 1.9 for the PP-side and the PVDF-side, respectively. As shown in the figure, the median VAS scores for both sides increased more than twice on the first day after surgery and decreased within the first weak reaching almost the preoperative median VAS scores. At the 3-month follow-up median VAS scores for both sides at least halved (PP-side 0.9, PVDF-side 0.7) compared to the preoperative scores. Afterwards, median VAS scores decreased continually till the last follow-up at one year. Significantly lower VAS scores were recorded at the 3-month (p < 0.05) and 6-month (p < 0.05) follow-up for the PVDF-side. At 1 year follow-up the median VAS score for the PVDF-side was half of the PP-side, however it was not statistically significant because of a very low pain level (VAS 0.2 vs 0.4) overall.
DISCUSSION
Despite the progress made in reducing recurrence and pain after inguinal hernia repair these complications are still the major challenges which need to be overcome [1]. Both recurrence and pain are multifactorial problem areas: Patients biological diversity as well as the surgical technique [16,17], are crucial outcome influencing factors. Especially, nerve management [18], and fixation [19], strongly influence pain.The two remaining Figure 1 Study flow-chart. factors are the polymer (PP vs. PVDF) and the porosity (49%vs. 73%). The study design does not allow investigating one of these two factors individually.The present study design aims to limit the multifactorial problem to a minimum of remaining influencing factors. In this respect, the most effective measures are a standardized surgical procedure, a randomization and the use of both meshes, which are compared, in the same patient. Focus of the present study was to investigate the influence of the used mesh implant. However, the “mesh implant” itself poses a multifactorial issue. The two major mesh properties which influence the clinical performance are the used raw-material (polymer) and the mesh design (porosity, filament structure (mono- vs. multifilament) and mechanical strength). The two mesh implants used in the study were chosen with the goal to limit these factors to a minimum.According to the international groin hernia guidelines a tensile strength of 16 N/cm fulfils the requirement of a mesh implant used in groin hernia repair. Regarding this criterion both meshes used for this study fulfil this requirement. Thus, mechanical strength should not present an influencing factor in this study. Neither should the filament structure have an influence, since both meshes are made from monofilaments with approximately the same thickness (PVDF: 130 µm vs. PP: 140 µm). The two remaining factors are the polymer (PP vs. PVDF) and the porosity (49% vs. 73%). The study design does not allow investigating one of these two factors individually.
In terms of the early postoperative complication (7 days after surgery) foreign body sensation was significantly higher on the PP-side compares to the PVDF-side. Each mesh implantation induces a foreign-body reaction leading to an encapsulation of the polymer filaments by a granuloma of inflammatory and fibrotic cells which is finally related to scar formation [1]. It is known that both polymer and porosity affect the granuloma size and respectively scar formation. In contrast to PVDF, PP triggers a higher foreign body reaction [7,8]. Regarding porosity it is known that small pore meshes foster the bridging effect [20], which leads excessive scar formation combined with higher foreign body sensation [21]. In case of the PP mesh implants used in this study, both described effects sum up. Therefore, the results of the study regarding foreign body sensation are in good accordance with published evidence about foreign body reaction and porosity.
At the 3 and 6 months follow-up the PP-side shows significantly higher late postoperative pain and chronic pain than the PVDF-side. Re-innervation and neo-innervation are known to occur following hernia repairs in indigenous tissue as well as through mesh implants. Bendavid et al., could show that mesh innervation is significantly higher for chronic pain patients [22]. As a consequence, innervation in none-indigenous tissue like granuloma and scar tissue, is the problem. Thus, it might be assumed that pronounced scar formation fosters pain and chronic pain. Taking these considerations into account, the explanation for the observed higher incidence of pain on the PP-side is based on the same principles as discussed above: a polymer with lower biocompatibility which triggers a higher foreign body reaction in combination with a small pore mesh that leads to an excessive scar formation. In consequence these two factors combined lead to a higher incidence of pain. These findings are confirmed by other studies using Lichtenstein and comparing small and large pore meshes [23-25]. Also these studies show advantages for large pore meshes during the first postoperative weeks and 3 months with regard to pain.
CONCLUSION
Analysis of the primary and secondary outcome parameters of this study show that both meshes (DynaMesh-LICHTENSTEIN and Surgipro) can be safely used for inguinal hernia repair. However, the PVDF-mesh performed considerably better in terms of immediate foreign body sensation (up to 7 days post surgery), late postoperative pain and chronic pain, which influences patients’ quality of life directly. We also recorded fewer recurrences over the full follow-up period for the PVDFmesh. Because of these findings, we conclude that PVDF large pore meshes are preferable for the Lichtenstein repair compared to PP small pore meshes.
A five-year follow-up might be considered to get valid insights about the course of pain as well as the long-term recurrence rate