The Causal Relationship between Four Different Smoking Behaviors and Carpal Tunnel Syndrome: A Mendelian Randomization Study
- 1. Department of Bone and Joint Surgery, Affiliated Hospital of Southwest Medical University, China
Abstract
Background: Previous observational studies have explored the causal relationship between smoking behavior and carpal tunnel syndrome (CTS), but these studies were affected by confounding factors and yielded inconsistent results. Therefore, further exploration and validation are still required to understand the causal relationship between smoking behavior and CTS.
Methods: We considered four smoking behaviors (past smoking, current smoking, smoking initiation, and never smoking) as exposures and CTS as the outcome. Two-sample Mendelian randomization analysis was employed to explore the causal relationships between these four exposures and the outcome. Exposure and outcome datasets were obtained from the IEU (Integrative Epidemiology Unit) Open genome-wide association study (GWAS) project (https:// gwas.mrcieu.ac.uk/). We conducted causal analysis using five methods (inverse-variance weighted method (IVW), weighted median method, MR Egger method, Simple mode, and weighted mode) before and after outlier exclusion. In this study, the IVW method was considered as the primary method for causal analysis. And the analysis results obtained after outlier exclusion were considered as the final results. Additionally, sensitivity analysis was performed to evaluate the robustness of the findings.
Results: The analysis results revealed that current smoking (IVW: OR: 2.485, 95% CI: 1.514-4.080, P=3.184×10-4) and smoking initiation (OR: 1.362, 95% CI: 1.193-1.555, P=4.626×10-6) remained as risk factors for CTS. On the contrary, past smoking (OR: 0.753, 95% CI: 0.635-0.893, P=0.001) and never smoking (OR: 0.410, 95% CI: 0.270-0.624, P=3.056×10-5) continued to exhibit protective effects against CTS.
Conclusion: Our study identified a significant association between current smoking and smoking initiation, which were found to increase the risk of CTS. Conversely, past smoking and never smoking were significantly associated with a decreased risk of CTS. These novel findings hold potential clinical significance as they can inform the development of targeted interventions for specific populations. This, in turn, can enable early prevention of CTS and facilitate timely detection and treatment for individuals with CTS.
KEYWORDS
- Past smoking
- Current smoking
- Smoking initiation
- Never smoking
- Carpal Tunnel Syndrome (CTS)
- Mendelian Randomization (MR)
CITATION
Zhao L, Zheng X, Fei L, Shen J, Xu X, Ye J (2024) The Causal Relationship between Four Different Smoking Behaviors and Carpal Tunnel Syndrome: A Mendelian Randomization Study. Ann Sports Med Res 11(2): 1229.
ABBREVIATIONS
CTS: Carpal Tunnel Syndrome; MR: Mendelian Randomization; IEU: Integrative Epidemiology Unit; GWAS: Genome-Wide Association Study; IVW: Inverse Variance Weighted; OR: Odds Ratio; CI: Confidence Interval; IVs: Instrumental Variables; Chr: Chromosome; A1: Effect Allele; A2: Other Allele; EAF: Effect Allele Frequency; SE: Standard Error.
BACKGROUND
Carpal tunnel syndrome (CTS) is a clinical condition caused by the compression of the median nerve below the transverse carpal ligament in the wrist [1,2]. It is commonly characterized by symptoms such as hand pain, numbness, and discomfort, ultimately leading to limited functional activities of the hand [1]. CTS is not only the most common peripheral nerve compression syndrome [3,4], but also one of the most common musculoskeletal disorders of the upper limb [5]. The prevalence of CTS varies among different populations, ranging from 1-5% in the general population [6-9] to as high as 21% in the working population [10]. This high prevalence makes it considered as the most costly musculoskeletal disorder of the upper limb [11], with Stapleton et al. [12], estimating annual medical costs for CTS in the United States to exceed 2 billion dollars. Therefore, CTS not only has serious physical consequences for patients, but can also result in significant economic burden [2]. Smoking has been associated with the occurrence and progression of various diseases, posing many hazards to health [13,14]. In recent years, numerous studies have investigated the relationship between smoking and CTS [15,16]. However, the results have been inconsistent, and most of the previous studies were observational in nature, with many confounding factors and reverse causality interference, making it impossible to establish causal inference. Therefore, further exploration and validation are needed to understand the causal relationship between smoking and CTS.
Mendelian Randomization (MR) is a novel genetic epidemiological research design that utilizes Single Nucleotide Polymorphisms (SNPs) as Instrumental Variables (IVs). It effectively addresses common limitations in traditional observational studies, such as confounding, reverse causality, and measurement errors, enabling causal inference [17]. However, to the best of our knowledge, no study has yet investigated the causal relationship between smoking behaviors and Carpal Tunnel Syndrome (CTS) using MR. Therefore, the primary objective of our study is to employ MR methodology to further explore the causal relationship between different smoking behaviors and CTS, with the aim of providing valuable guidance for clinical practice.
METHODS AND DATA
Study Design
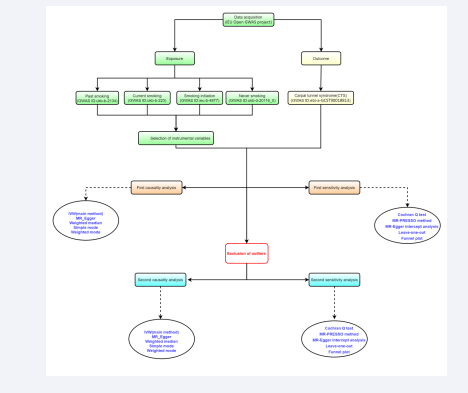
In this study, we consider four distinct smoking behaviors (including past smoking, current smoking, smoking initiation, and never smoking) as exposure factors, with Carpal Tunnel Syndrome (CTS) as the outcome factor. We will perform Mendelian Randomization (MR) analysis using Genome-Wide Association Study (GWAS) data on exposures and outcomes obtained from publicly available databases. The detailed workflow for the study design is illustrated in Figure 1.
Figure 1: The study design. IVW: Inverse Variance Weighted; IEU: Integrative Epidemiology Unit; GWAS: Genome-Wide Association Study.
Data Source
All GWAS data on exposures and outcome are obtained from the Integrative Epidemiology Unit (IEU) Open GWAS project (https://GWAS.mrcieu.ac.uk/). More information on exposure and outcome can be found in Table 1.This database initially served as the base database for the MR-Base and LD Hub projects and primarily includes publicly available datasets. The data includes both males and females, and the ultimate population source is European. Since the data used in this study are publicly available, anonymous, and de-identified, ethical approval from an ethics review board is not required for this study.
Selection of IVs
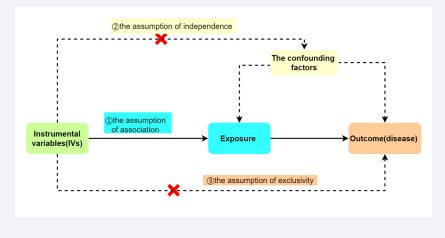
In this study, Single Nucleotide Polymorphisms (SNPs) are used as Instrumental Variables (IVs) to investigate the causal relationship between exposures and outcomes. SNPs associated with exposures (past smoking, current smoking, smoking initiation, and never smoking) are extracted from the IEU Open GWAS project (https://GWAS.mrcieu.ac.uk/). The selection of IVs must satisfy three fundamental assumptions for MR analysis [Figure 2]:
Figure 2: Three fundamental assumptions of MR.
1. The close correlation between the IVs and exposure
2. The IVs should not be correlated with confounding factors that affect the exposure and the disease
3. The IVs can only affect the disease through the exposure, without any other pathways
the assumption of association, the assumption of independence, and the assumption of exclusivity. To ensure that the selected IVs meet the three assumptions, we implemented the following measures. Firstly, to fulfill the assumption of association, we applied the following criteria: SNPs closely associated with past smoking, smoking initiation, and never smoking were selected based on P<5×10-8, clustering window>10,000kb, and linkage disequilibrium level (r2<0.001). It should be noted that to obtain more SNPs associated with current smoking, we set the threshold at P<5×10-7, because prior research has already established the threshold of P<5×10-5 in order to identify additional single nucleotide polymorphisms (SNPs) that are correlated with heightened exposure [18]. An F-statistic greater than 10 was considered to indicate a strong association between IVs and exposures [19]. Therefore, we computed the F-value for each SNP, excluding SNPs with F≤10. The F-value was calculated using the formula F=beta2/se2 (where beta represents the estimated effect of the SNP and se denotes the standard error of the beta value). Secondly, to satisfy the assumption of independence, we searched for each SNP in the Human Genotype-Phenotype Database (http://www. phenoscanner.medschl.cam.ac.uk/). If a SNP was found to be associated with confounding factors that influence the studied exposure and disease, it was excluded. Thirdly, to achieve the assumption of exclusivity, a P<5×10-8 was considered as evidence of relevance, and if a SNP was found to be associated with the outcome (P<5×10-8), it was excluded.
Method selection for establishing causal relationships
In this study, we employed five methods for causal relationship analysis in the random-effects model, namely the Inverse-Variance Weighting method (IVW), weighted median method, MR Egger method, Simple mode, and Weighted mode. The IVW model is considered the most robust method for causal inference in MR analysis of two samples [20]. Therefore, we selected the IVW method as the primary method for determining the presence of causal relationships in this study. If the P-value obtained from the IVW analysis is less than 0.05, we consider there to be a potential causal relationship between the exposure and outcome, and determine whether the exposure is a risk factor or protective factor based on the magnitude of the Odds Ratio (OR).
Sensitivity Analysis
Multiple methods were employed for sensitivity analysis. Firstly, we assessed the heterogeneity of the IVW model using the Cochran’s Q test, where a P-value less than 0.05 indicates the presence of heterogeneity. However, the presence of heterogeneity does not necessarily render the IVW model ineffective [19]. Secondly, we employed the MR-PRESSO method to detect outliers. If any outliers were identified, they were immediately removed and the analysis was re-performed. Thirdly, the MR-Egger intercept analysis was utilized to test for pleiotropy of the SNP. A P-value less than 0.05 indicates significant pleiotropy in the MR analysis. Fourthly, we assessed the stability of the results using the leave-one-out method. If the removal of a specific SNP had a substantial impact on the results, it was removed and the analysis was re-conducted. Lastly, we visually presented the results of the sensitivity analysis by plotting a funnel plot.
Statistical Analysis
All statistical analyses in this study were conducted using the R software package, including the two-sample MR and MR- PRESSO analyses [21]. Both of these packages are integrated within the R software (version 4.3.1).
RESULTS
Selection of IVs
For the four exposures (past smoking, current smoking, smoking initiation, and never smoking), we conducted a rigorous hierarchical screening based on the three core assumptions of MR. Ultimately, we included 94, 64, 85, and 76 SNPs, respectively. Each SNP had an F-statistic greater than 10, indicating the absence of weak instrument bias. For further information about each SNP, please refer to [Tables S1, S2, S3, and S4].
First causal Analysis
We conducted the initial causal analysis between each exposure, as determined by the SNP corresponding to each exposure after rigorous screening based on the three core assumptions of MR, and the outcome (CTS). The results of the analysis using the five methods are presented in Figure S1. The results indicated significant associations between all four exposures (past smoking, current smoking, smoking initiation, and never smoking) and CTS (IVW method: all P-values were less than 0.05). Specifically, the results showed that current smoking (IVW analysis, OR: 2.291, 95% CI: 1.359-3.862, P=0.002) and initiation of smoking (OR: 1.367, 95% CI: 1.190-1.572, P=1.081×10-5) were risk factors for CTS. In contrast, previous smoking (OR: 0.733, 95% CI: 0.614-0.874, P=0.001) and never smoking (OR: 0.382, 95% CI: 0.246-0.593, P=1.828×10-5) were protective factors for CTS.
First Sensitivity Analysis
Heterogeneity testing was conducted using the IVW method, and the results indicated significant heterogeneity for all four exposures (past smoking, current smoking, smoking initiation, and never smoking) (Cochran Q test: all P-values were less than 0.05). Table S5 provides additional information, and a funnel plot was generated to visually represent the heterogeneity results,as shown in Figure S2. MR-PRESSO was employed to identify outliers, and it was found that each exposure had outliers: one outlier for past smoking (rs74676797), one outlier for current smoking (rs11210229), two outliers for smoking initiation (rs6728726, rs7555507), and one outlier for never smoking (rs7567570). The MR-Egger intercept test did not indicate the presence of pleiotropy for the SNPs corresponding to each exposure (P>0.05). Leave-one-out analysis demonstrated the stability of the results. For example, we can see in fig.S3 the results of first analysis current smoking and CTS using the leave- one-out.
Second Causal Analysis
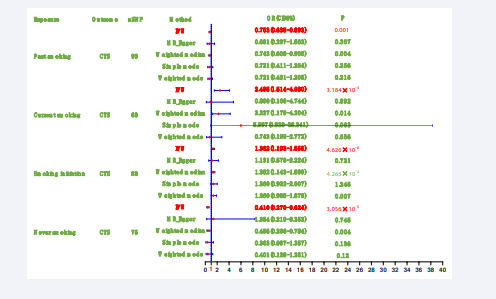
Considering the identification of outliers by MR-PRESSO, we conducted a second round of causal analysis by excluding the outliers associated with each exposure. The results of this analysis were considered as the final findings for the causal relationships. The outcomes of the five causal analysis methods are presented in Figure 3.
Figure 3: Summary of the results of the second causal analysis.
After excluding the outliers, the results demonstrated that current smoking (OR: 2.485, 95% CI: 1.514-4.080, P=3.184×10-4) and smoking initiation (OR: 1.362, 95% CI: 1.193-1.555, P=4.626×10-6) remained as risk factors for CTS. Conversely, past smoking (OR: 0.753, 95% CI: 0.635-0.893, P=0.001) and never smoking (OR: 0.410, 95% CI: 0.270-0.624, P=3.056×10-5) continued to exhibit protective effects against CTS. These results indicate a consistent direction of findings between the first and second analyses.
Second Sensitivity Analysis
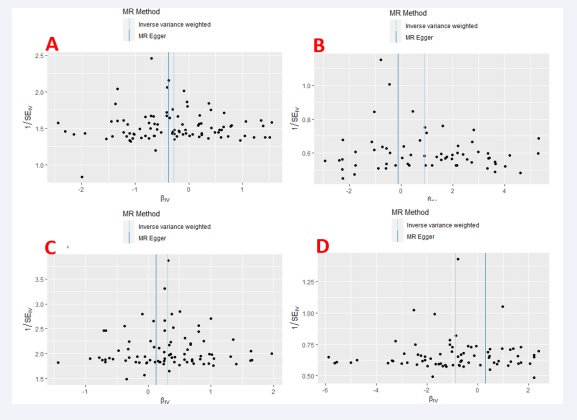
Similarly, after excluding the outliers, the Cochran Q test indicated that the SNPs corresponding to the four exposures still exhibited heterogeneity. However, this does not imply that our analysis results are meaningless. We also generated funnel plots to visualize the heterogeneity results, as shown in Figure 4.
Figure 4: Funnel plot of four smoking behaviors and carpal tunnel syndrome. (A) Past smoking; (B) Current smoking; (C) Smoking initiation; (D) Never smoking.
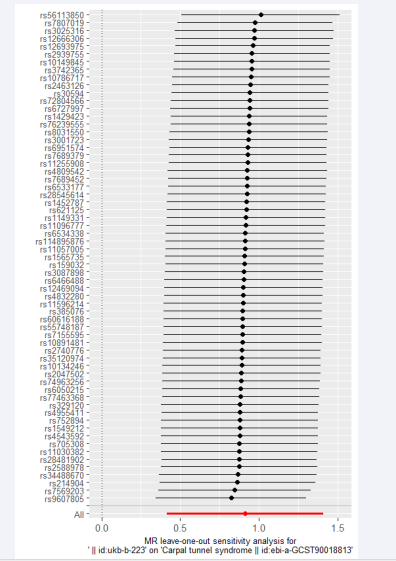
Furthermore, we conducted another round of outlier detection using MR-PRESSO, but no outliers were identified. The MR-Egger intercept test did not reveal evidence of horizontal pleiotropy. Leave-one-out analysis also demonstrated the stability of the results. For example, we can see in Figure 5 the results of second analysis current smoking and CTS using the leave-one-out.
Figure 5: The results of the second analysis using leave-one-out method on current smoking and carpal tunnel syndrome.
DISCUSSION
In this study, we investigated the causal relationships between four smoking behaviors (past smoking, current smoking, smoking initiation, and never smoking) and the risk of CTS using two-sample MR analysis. The results from the second round of causal analysis were considered as the final outcomes of this study. Our analysis revealed that current smoking and smoking initiation are associated with an increased risk of CTS. Conversely, past smoking and never smoking are associated with a decreased risk of CTS.
Prior to this study, several investigations have examined the relationship between smoking and CTS. Hulkkonen et al. [15], conducted a 31-year follow-up study on a general working population in northern Finland and found that smokers had an increased risk of CTS hospitalization (HR 1.48, 95% CI 1.12- 1.96). Guan et al. [22], conducted a case-control study with 1512 CTS outpatients as cases and 4536 non-CTS outpatients as controls, and identified smoking (OR: 4.862, 95% CI: 3.991- 5.925) as a risk factor for CTS. Hulkkonen et al. [23], conducted a birth cohort study with a sample size of 8703 and found an association between smoking among offspring and CTS risk, while maternal smoking was not associated with an increased risk of CTS in offspring. Roquelaure et al. [24], conducted a cross-sectional study on 711 male workers aged 30-65 years engaged in farming or agricultural work in a French mutual agricultural and farmer fund-covered cohort, and found that current smoking (OR=2.1, 95% CI: 1.0-4.5) was associated with an increased risk of CTS. Furthermore, a recent cross-sectional study also identified smoking (OR: 4.2, 95% CI: 1.76-10.26) as an independent risk factor for CTS [25]. All of these studies suggest that smoking is a risk factor for CTS. However, there have been a few studies that found no association between smoking and CTS. Geoghegan et al. [16], conducted a large case-control study using the UK General Practice Research Database and found that smoking was not a risk factor for CTS after multivariate analysis. Additionally, a meta-analysis of case-control studies found no association between smoking and CTS [26]. Overall, previous studies have explored the relationship between smoking and CTS, and although there are some discrepancies in the results, the majority of studies still support smoking as a risk factor for CTS. The mechanisms underlying this association have been sparsely investigated. Rinker et al. [27], demonstrated in an animal model that smoking has detrimental effects on sciatic nerve blood flow, ultimately leading to nerve degeneration and fibrosis, which may be one of the reasons. Additionally, a study that assessed CTS from multiple angles found that smoking may be associated with CTS occurrence through microcirculation impairment [28]. Both studies suggest that impaired blood flow may be involved in the mechanism, but further research is needed to explore and validate these findings.
Although previous research has explored the relationship between smoking and CTS, the results have been inconsistent, and most of the previous studies have investigated the relationship between smoking and CTS in a general manner without further examining specific smoking behaviors. In contrast, we categorized smoking behaviors into four types: past smoking, current smoking, smoking initiation, and never smoking. We employed MR, a novel epidemiological analysis method, for the first time to minimize the impact of confounding factors [29]. Our study findings demonstrated that current smoking and smoking initiation are associated with an increased risk of CTS, while never smoking is associated with a decreased risk of CTS, which is consistent with the majority of previous research. However, it is worth noting that our study surprisingly found that past smoking is a protective factor for CTS. We are currently unable to explain this result and further research is needed for validation and interpretation. Based on the results of this study, there are implications for our clinical practice. Firstly, we should strengthen the awareness of the harmful effects of smoking and actively encourage individuals who currently smoke or are starting to smoke to quit as soon as possible in order to reduce the incidence of CTS. Secondly, we should enhance screening for CTS in individuals who currently smoke or are starting to smoke, allowing for early detection and treatment, which can lead to better treatment outcomes and prognosis for patients.
STRENGTHS AND LIMITATIONS
This study has both strengths and limitations. The strengths are primarily reflected in the following aspects. Firstly, MR analysis effectively mitigates inherent limitations commonly encountered in traditional observational studies, such as confounding bias, reverse causality, and measurement errors [17]. Secondly, sensitivity and multi-effect analyses were conducted to ensure the accuracy of the MR analysis. Thirdly, to minimize potential bias, we included diverse European populations from different countries as the exposure and outcome groups. Fourthly, we have further categorized smoking behavior into four distinct types, enhancing the specificity and epidemiological significance of our research findings. The limitations are as follows. Firstly, the F-statistic results indicate a very good fit of the instrumental variables used in this study with the exposure (F-statistic greater than 10). However, most F-statistic values are below 100, which may affect the accuracy of the results [19]. Secondly, the GWAS data used in this study were not stratified by gender, thus precluding gender-specific stratified analysis [19].
CONCLUSION
In conclusion, this study employed the MR to investigate the causal relationships between four different smoking behaviors (past smoking, current smoking, smoking initiation, and never smoking) and CTS. The results demonstrated a significant association between current smoking and smoking initiation with an increased risk of CTS, while past smoking and never smoking were significantly associated with a decreased risk of CTS. These novel findings have potential clinical significance, as they can guide the implementation of targeted interventions for corresponding populations, enabling early prevention of CTS and facilitating early detection and timely treatment for CTS patients.
DECLARATIONS
Ethical Approval and Consent to Participate
Since the data used in this study are publicly available, anonymous, and de-identified, ethical approval from an ethics review board is not required for this study.
AVAILABILITY OF SUPPORTING DATA
All data used in this study were obtained from the Integrative Epidemiology Unit (IEU) Open GWAS project (https://GWAS. mrcieu.ac.uk/). The data utilized for the final analysis can be found in this manuscript and its supplementary materials.
AUTHORS CONTRIBUTIONS
Liang Zhao has contributed in the areas of methodology, data organization and analysis, result visualization, writing the manuscript, writing review and editing. Xuzhou Zheng, Lincong Fei, Juncheng Shen and Xuepeng Xu participated in literature searching, formal analysis, writing review, and editing. Junwu Ye contributed in data management, project management, supervision, resource management, as well as writing review and editing. All authors have made contributions to the article and have approved the submitted version.
ACKNOWLEDGMENTS
We would like to express our deep appreciation to the Integrative Epidemiology Unit (IEU) Open GWAS project. We are grateful to the project team for their diligent efforts in curating the relevant data, which was indispensable for conducting the analysis in this study.
REFERENCES
- Verdugo RJ, Salinas RA, Castillo JL, Cea JG. Surgical versus non- surgical treatment for carpal tunnel syndrome. Cochrane Database Syst Rev. 2008; 2008: CD001552.
- Burton CL, Chen Y, Chesterton LS, van der Windt DA. Trends in the prevalence, incidence and surgical management of carpal tunnel syndrome between 1993 and 2013: an observational analysis of UK primary care records. BMJ Open. 2018; 8: e020166.
- Padua L, Coraci D, Erra C, Pazzaglia C, Paolasso I, Loreti C, et al. Carpal tunnel syndrome: clinical features, diagnosis, and management. Lancet Neurol. 2016; 15: 1273-1284.
- A Sousa LH, O Costa C, Novak EM, Giostri GS. Complex Regional Pain Syndrome after Carpal Tunnel Syndrome Surgery: A Systematic Review. Neurol India. 2022; 70: 491-503.
- Calandruccio JH, Thompson NB. Carpal Tunnel Syndrome: Making Evidence-Based Treatment Decisions. Orthop Clin North Am. 2018; 49: 223-229.
- Tanaka S, Wild DK, Seligman PJ, Halperin WE, Behrens VJ, Putz- Anderson V. Prevalence and work-relatedness of self-reported carpal tunnel syndrome among U.S. workers: analysis of the Occupational Health Supplement data of 1988 National Health Interview Survey. Am J Ind Med. 1995; 27: 451-470.
- Stevens JC, Sun S, Beard CM, O’Fallon WM, Kurland LT. Carpal tunnel syndrome in Rochester, Minnesota, 1961 to 1980. Neurology. 1988;38: 134-138.
- de Krom MC, Knipschild PG, Kester AD, Thijs CT, Boekkooi PF, Spaans F. Carpal tunnel syndrome: prevalence in the general population. J Clin Epidemiol. 1992; 45: 373-376.
- Atroshi I, Gummesson C, Johnsson R, Ornstein E, Ranstam J, Rosen I. Prevalence of carpal tunnel syndrome in a general population. JAMA. 1999; 282: 153-158.
- Gorsche RG, Wiley JP, Renger RF, Brant RF, Gemer TY, Sasyniuk TM. Prevalence and incidence of carpal tunnel syndrome in a meat packing plant. Occup Environ Med. 1999; 56: 417-422.
- Dale AM, Harris-Adamson C, Rempel D, Gerr F, Hegmann K, Silverstein B, et al. Prevalence and incidence of carpal tunnel syndrome in US working populations: pooled analysis of six prospective studies. Scand J Work Environ Health. 2013; 39: 495-505.
- Stapleton MJ. Occupation and carpal tunnel syndrome. ANZ J Surg. 2006; 76: 494-496.
- Pietinalho A, Pelkonen A, Rytila P. Linkage between smoking and asthma. Allergy. 2009; 64: 1722-1727.
- Schroeder SA. Public smoking bans are good for the heart. J Am Coll Cardiol. 2009; 54: 1256-1257.
- Hulkkonen S, Shiri R, Auvinen J, Miettunen J, Karppinen J, Ryhanen J. Risk factors of hospitalization for carpal tunnel syndrome among the general working population. Scand J Work Environ Health. 2020; 46: 43-49.
- Geoghegan JM, Clark DI, Bainbridge LC, Smith C, Hubbard R. Risk factors in carpal tunnel syndrome. J Hand Surg Br. 2004; 29: 315-320.
- Zhou H, Zhang Y, Liu J, Yang Y, Fang W, Hong S, et al. Education and lung cancer: a Mendelian randomization study. Int J Epidemiol. 2019; 48: 743-750.
- Sanna S, van Zuydam NR, Mahajan A, Kurilshikov A, Vich Vila A, Vosa U, et al. Causal relationships among the gut microbiome, short-chain fatty acids and metabolic diseases. Nat Genet. 2019; 51: 600-605.
- Yang W, Yang Y, He L, Zhang M, Sun S, Wang F, et al. Dietary factors and risk for asthma: A Mendelian randomization analysis. Front Immunol. 2023; 14: 1126457.
- Hartwig FP, Davey Smith G, Bowden J. Robust inference in summary data Mendelian randomization via the zero modal pleiotropy assumption. Int J Epidemiol. 2017; 46: 1985-1998.
- Long Y, Tang L, Zhou Y, Zhao S, Zhu H. Causal relationship between gut microbiota and cancers: a two-sample Mendelian randomisation study. Bmc Med. 2023; 21: 66.
- Guan W, Lao J, Gu Y, Zhao X, Rui J, Gao K. Case-control study on individual risk factors of carpal tunnel syndrome. Exp Ther Med. 2018; 15: 2761-2766.
- Hulkkonen S, Auvinen J, Miettunen J, Karppinen J, Ryhanen J. Smoking as risk factor for carpal tunnel syndrome: A birth cohort study. Muscle Nerve. 2019; 60: 299-304.
- Roquelaure Y, Jego S, Geoffroy-Perez B, Chazelle E, Descatha A, Evanoff B, et al. Carpal Tunnel Syndrome Among Male French Farmers and Agricultural Workers: Is It Only Associated With Physical Exposure? Saf Health Work. 2020; 11: 33-40.
- Demissie B, Yenew C, Alemu A, Bantie B, Sume BW, Deml YA, et al. Carpal tunnel syndrome and its associated factors among computer user bankers in South Gondar Zone, Northwest Ethiopia, 2021: a cross sectional study. BMC Musculoskelet Disord. 2023; 24: 828.
- Pourmemari MH, Viikari-Juntura E, Shiri R. Smoking and carpal tunnel syndrome: a meta-analysis. Muscle Nerve. 2014; 49: 345-350.
- Rinker B, Fink BF, Barry NG, Fife JA, Milan ME, Stoker AR, et al. The effect of cigarette smoking on functional recovery following peripheral nerve ischemia/reperfusion injury. Microsurgery. 2011; 31: 59-65.
- Padua L, Aprile I, Caliandro P, Carboni T, Meloni A, Massi S, et al. Italian Carpal Tunnel Syndrome Study G. Symptoms and neurophysiological picture of carpal tunnel syndrome in pregnancy. Clin Neurophysiol. 2001; 112: 1946-1951.
- Skrivankova VW, Richmond RC, Woolf BAR, Davies NM, Swanson SA, VanderWeele TJ, et al. Strengthening the reporting of observational studies in epidemiology using mendelian randomisation (STROBE- MR): explanation and elaboration. BMJ. 2021; 375: n2233.